Intralobar fibres of the occipital lobe: a post mortem dissection study
1
2014
... 浅表白质(superficial white matter,SWM)位于大脑皮质下的轴突层,它主要由短关联纤维或U型纤维组成,这些纤维连接着由一个或几个皮质褶皱分隔开的邻近皮质区域[1].SWM是大脑最后形成髓鞘的组织区域,因此更能表现出大脑成熟过程中发生的损害和变化[2].最近的一些研究表明,SWM纤维与多种大脑疾病密切相关,例如:自闭症、精神分裂症、脑炎、癫痫、帕金森病和脑小血管病,并且在构建完整人类连接组以及解释人类大脑功能上,SWM成像研究有着重要意义[3].尽管SWM的纤维连接数量大约是深部白质(deep white matter,DWM)的两倍,但由于SWM尺寸小、结构复杂性高、高度弯曲,以及皮层折叠的高度变异性,对SWM纤维束的提取上存在一定困难[4]. ...
Superficial white matter fiber systems impede detection of long-range cortical connections in diffusion MR tractography
1
2015
... 浅表白质(superficial white matter,SWM)位于大脑皮质下的轴突层,它主要由短关联纤维或U型纤维组成,这些纤维连接着由一个或几个皮质褶皱分隔开的邻近皮质区域[1].SWM是大脑最后形成髓鞘的组织区域,因此更能表现出大脑成熟过程中发生的损害和变化[2].最近的一些研究表明,SWM纤维与多种大脑疾病密切相关,例如:自闭症、精神分裂症、脑炎、癫痫、帕金森病和脑小血管病,并且在构建完整人类连接组以及解释人类大脑功能上,SWM成像研究有着重要意义[3].尽管SWM的纤维连接数量大约是深部白质(deep white matter,DWM)的两倍,但由于SWM尺寸小、结构复杂性高、高度弯曲,以及皮层折叠的高度变异性,对SWM纤维束的提取上存在一定困难[4]. ...
Imaging of the superficial white matter in health and disease
1
2024
... 浅表白质(superficial white matter,SWM)位于大脑皮质下的轴突层,它主要由短关联纤维或U型纤维组成,这些纤维连接着由一个或几个皮质褶皱分隔开的邻近皮质区域[1].SWM是大脑最后形成髓鞘的组织区域,因此更能表现出大脑成熟过程中发生的损害和变化[2].最近的一些研究表明,SWM纤维与多种大脑疾病密切相关,例如:自闭症、精神分裂症、脑炎、癫痫、帕金森病和脑小血管病,并且在构建完整人类连接组以及解释人类大脑功能上,SWM成像研究有着重要意义[3].尽管SWM的纤维连接数量大约是深部白质(deep white matter,DWM)的两倍,但由于SWM尺寸小、结构复杂性高、高度弯曲,以及皮层折叠的高度变异性,对SWM纤维束的提取上存在一定困难[4]. ...
Superficial white matter imaging: Contrast mechanisms and whole-brain in vivo mapping
1
2020
... 浅表白质(superficial white matter,SWM)位于大脑皮质下的轴突层,它主要由短关联纤维或U型纤维组成,这些纤维连接着由一个或几个皮质褶皱分隔开的邻近皮质区域[1].SWM是大脑最后形成髓鞘的组织区域,因此更能表现出大脑成熟过程中发生的损害和变化[2].最近的一些研究表明,SWM纤维与多种大脑疾病密切相关,例如:自闭症、精神分裂症、脑炎、癫痫、帕金森病和脑小血管病,并且在构建完整人类连接组以及解释人类大脑功能上,SWM成像研究有着重要意义[3].尽管SWM的纤维连接数量大约是深部白质(deep white matter,DWM)的两倍,但由于SWM尺寸小、结构复杂性高、高度弯曲,以及皮层折叠的高度变异性,对SWM纤维束的提取上存在一定困难[4]. ...
Brain fiber structure estimation based on principal component analysis and RINLM filter
1
2024
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Tracking neuronal fiber pathways in the living human brain
1
1999
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Occipito-temporal connections in the human brain
3
2003
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
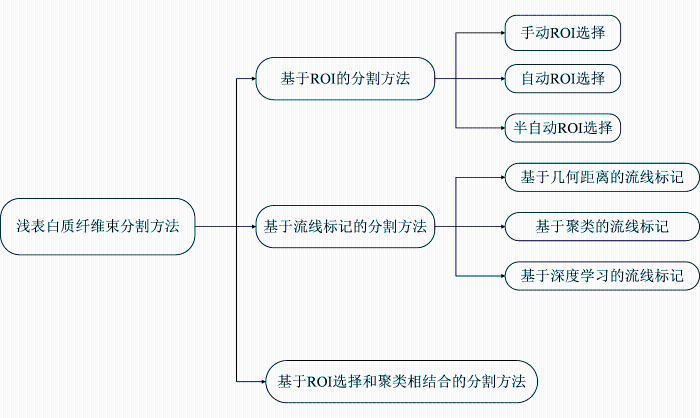
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
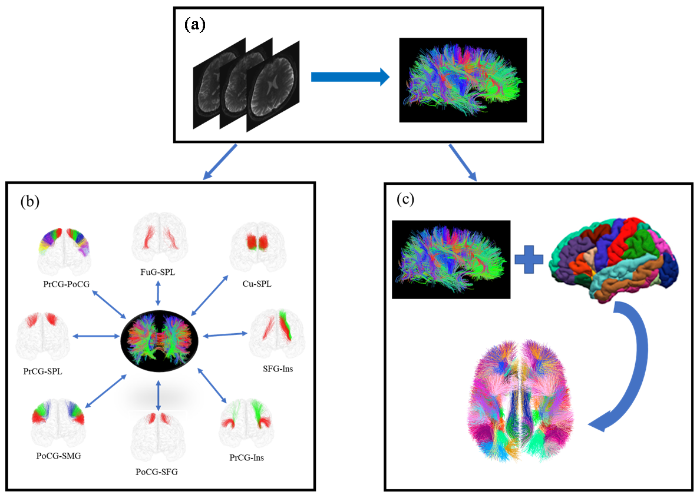
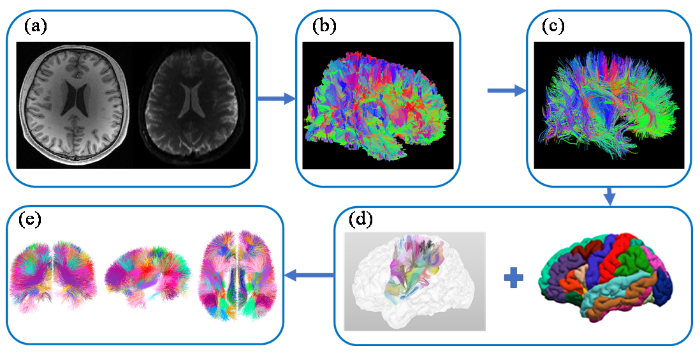
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Segmentation of short association bundles in massive tractography datasets using a multi-subject bundle atlas
1
2011
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Supwma: consistent and efficient tractography parcellation of superficial white matter with deep learning
1
2022
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Global white matter tractography using swarm optimization
1
2012
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
全局脑白质纤维群智能跟踪算法
1
2012
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
1
2017
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
A fiber tracking algorithm based on non-local constrained spherical deconvolution
1
2020
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
基于非局部约束球面反卷积模型的纤维追踪算法
1
2020
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Characterization of U-shape streamline fibers: methods and applications
3
2014
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
... 为了减少每个流线的标记量,许多流线标记方法首先将流线分组为簇,然后为每个簇分配解剖标签,这种方法被称为聚类方法.Guevara等人[49]是第一个在纤维束聚类中涉及SWM工作的,他们的工作通过层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分组,提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM图谱还包含每个半球的47个SWM束.该研究主要对DWM进行聚类,并未对SWM进行针对性的研究.Zhang等人[13]的研究是第一个仅针对于SWM纤维束的研究工作,他们同样使用欧几里得距离进行纤维聚类,从DTI、HARDI、DSI数据中识别和表征U型纤维,证明了大多数U型纤维通过沿脑沟来连接相邻的脑回.Román等人[50]提出的方法是Guevara等人提出方法的改进,该方法专门针对短关联纤维进行聚类,并且只选择35~85 mm的短纤维,同时该方法还使用了Desikan-Killiany FreeSurfer皮质分区[51]自动命名生成SWM纤维束,最终通过74名健康受试者的高质量HARDI数据生成了一个包含左半球44个、右半球49个以及两个半球共有的33个SWM纤维束的图谱. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Tracing short connections of the temporo-parieto-occipital region in the human brain using diffusion spectrum imaging and fiber dissection
3
2016
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Anat-SFSeg: Anatomically-guided superficial fiber segmentation with point-cloud deep learning
3
2024
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
... 基于深度学习的流线标记分割方法是根据已有的纤维束分割数据训练模型,并预测新受试者中每个流线的解剖标签.现有的基于深度学习方法的纤维束分割方法大多数都是针对于DWM纤维束进行分割,很少有针对于SWM纤维束进行分割的方法.Xue等人[55]提出了一个名为Superficial White Matter Analysis(SupWMA)的新型两阶段深度学习框架,用于从全脑纤维束中高效且一致地分割198个SWM簇.该深度学习框架适应了基于点云的网络到SWM分割任务,在第二阶段使用监督对比学习,以增强SWM纤维束的特征学习,通过数据增强技术获得SWM合理纤维束和异常值之间更具区分性的表示.该研究是第一个通过深度学习来进行SWM纤维束分割的工作.Zhang等人[15]提出了一种新的基于解剖学引导的SWM纤维束分割框架,该框架引入了一种独特的纤维解剖学描述符,称为FiberAnatMap,它结合了个体和群体水平的解剖学特征,利用基于点云深度学习网络,将纤维的空间坐标和FiberAnatMap作为输入,提高了分割的准确性,用于改善深度学习网络对SWM纤维束的分割性能. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Research progress of neural fiber tracking
1
2020
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
神经纤维跟踪算法研究进展
1
2020
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
Surface-based probabilistic fiber tracking in superficial white matter
2
2024
... 扩散磁共振成像(diffusion magnetic resonance imaging,dMRI)作为一种非侵入性的成像技术,已成为研究神经解剖学和脑连接的重要工具,能够探测组织内水分子的扩散模式,从而揭示组织微结构信息[5].近年来,基于dMRI的浅表白质纤维成像研究有所增加.Conturo等人[6]是最早研究SWM纤维成像工作的研究者之一,他们使用纤维束追踪技术来识别白质(white matter,WM)束,包括一些U型纤维,该研究表明SWM纤维结构的各向异性低于DWM.Catani等人[7]通过放置感兴趣区域(region of interest,ROI)来研究SWM纤维束的连接,该研究证明了一条位于下纵束外侧U型纤维,连接大脑邻近的脑回.Guevara等人[8]通过聚类方法来研究SWM纤维束图谱的,该研究提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM束还包含每个半球的47个SWM束.Xue等人[9]通过深度学习来进行SWM纤维束的分割工作,该工作促进了深度学习在SWM纤维束研究的发展.在国内,纤维束追踪算法研究起步较晚,Feng等人[10]提出了一种群智能全局优化算法,该算法基于von Miser-fisher分布函数的信息素模型,通过信息素模型诱导迭代优化纤维轨迹.Zhang[11]提出一种脑纤维流线微分方程跟踪算法,在跟踪过程中搜索出邻域体素中与自身行进方向相近的多个纤维方向,建立一个三维空间上连续平滑的方向流场来表征纤维流线的分布,减小纤维建模误差带来的方向估计不准确的影响,通过龙格库塔数值积分方法求解流线微分方程,得到任意空间区域内整齐、平滑的纤维束集.Yue等人[12]提出了一种基于非局部约束球面反卷积模型的确定型纤维追踪算法,分数阶的非局部特性使得纤维方向分布模型估计的误差更小,而邻域信息的引入保证了空间一致性,可以减少噪声的影响,从而使得纤维束追踪更加准确.上述国内研究者提出的纤维束追踪算法都是针对于DWM,对于SWM的研究,国内的研究者也做出了巨大的贡献.Zhang等人[13]通过纤维聚类方法分别对人类、黑猩猩和猕猴大脑的扩散张量成像(diffusion tensor imaging,DTI)、高角度分辨率扩散成像(high angular resolution diffusion imaging,HARDI)和扩散光谱成像(diffusion spectrum imaging,DSI)数据进行U型纤维研究,他们验证了U型纤维的存在,并证明这些纤维通过围绕皮质沟来连接相邻的脑回.Wu等人[14]通过手动ROI分割方法在DSI数据上对颞叶、顶叶、枕叶的短纤维束进行分割,确定了三个纤维束即上纵束后段、垂直枕束和颞顶叶连接束,这些研究得到了纤维解剖技术的验证.Zhang等人[15]是国内第一个采用深度学习的方法对SWM进行分割的研究组,在他们这项工作中,提出了一种解剖引导的SWM分割框架(Anat-SFSeg)来提高SWM分割的性能.该框架由独特的纤维解剖图谱和基于点云的深度学习网络组成,该网络框架在所提供的数据集上实现了最高的分割准确度,并且在临床数据集上表现出了很好的泛化能力.上述SWM纤维研究都是依赖于现有的DWM纤维束追踪方法[16],没有一种方法专门应用于SWM纤维束.近几年,一些研究者提出了仅针对于SWM纤维束成像的算法[17],这些算法的提出推动了SWM纤维束分析和应用的发展. ...
... 由于SWM存在大量的高度弯曲纤维,现有的基于DWM纤维追踪算法不能重建出这种高度弯曲的纤维结构.为了解决该问题,Gahm等人[23]提出了一种基于表面的SWM纤维束追踪框架,该框架通过测量表面切空间上的角度变化,在三角网格内通过角度阈值控制路径的平滑度,并在跨三角网格时测量交叉角度,以确保追踪方向的准确性,这种方法利用了皮质表面的内在几何特性,更符合U型纤维沿着皮质表面的解剖学特性.与Gahm等人提出的确定性纤维束追踪算法不同,Nie等人[17]提出了一种新的基于表面的概率追踪框架,该框架将3D纤维取向分布(FOD)的球谐系数转换到每个三角形表面的局部坐标系中,从而将FOD投影到SWM的切空间,利用平行传输实现流线在SWM上的内在传播,根据概率抽样的纤维方向进行追踪,避免了传统基于体积追踪方法中必要的但具有挑战性的急剧转向.该算法重建了中央前回和中央后回的U型纤维,通过4个定量指标和MRtrix提供的基于体积的纤维束追踪算法以及基于表面的确定性追踪算法做对比,4个定量指标分别为重建纤维束的数量、U型纤维的连接完整性、U型纤维比率以及拓扑规律性.在重建纤维束的数量这个指标上,作者所提出的方法在所有三种方法中生成了最有效的连接,48.5%的种子点生成了有效连接.对于基于表面的确定性追踪算法,只有29.5%的种子点发展成有效连接.对于MRtrix的基于体积的纤维束追踪算法,只有6%的种子点生成有效的U型纤维连接.U型纤维的连接完整性是指将两个脑回的骨架均匀分成若干部分,统计被重建的纤维束是否在这些部分中,实验表明作者提出的算法重建出的U型纤维更完整.U型纤维比率是指两个端点之间的距离与纤维总长度的比值,较小的U型纤维比率表示成功重建有效的U型纤维,作者提出的算法相较于其他算法U型纤维比率是最低的.拓扑规律性是使用经典的多维标定方法将纤维束的起点和终点投影到二维平面,并计算它们之间的Procrustes距离,以评估重建纤维的拓扑规律性,通过计算作者提出的算法距离更短,这表明通过约束沿SWM表面的纤维追踪,可以生成形状更规则的U型纤维. ...
Superficial white matter: A review on the dMRI analysis methods and applications
1
2020
... 在基于dMRI的SWM纤维研究领域,以往研究仅对SWM传统分割方法和应用进行了概述[18],本文在此基础上加入了最新的基于深度学习SWM分割方法、SWM纤维束图谱构建和SWM纤维追踪新技术的研究进展.本文探讨的SWM纤维追踪技术未来有望成为该领域新的研究热点. ...
Pushing spatial and temporal resolution for functional and diffusion MRI in the Human Connectome Project
1
2013
... 在过去的十几年中,基于dMRI的大脑纤维束成像技术取得了巨大进展,dMRI的空间和角度分辨率显著提高[19].现有的大脑纤维束研究主要集中在DWM[20],为了构建完整人类连接组,一些研究者开始关注SWM纤维的连接.然而,现有的大脑纤维束成像技术都是针对于DWM来开发的,为了适应SWM的复杂纤维,一些研究者提出了专门针对于SWM纤维束连接的算法.这些算法考虑到了SWM高度弯曲、回旋偏差以及个体间皮层折叠模式高度变异性的特征,从而提高了SWM纤维束的成像质量.本节将会讨论如何从解决高度弯曲和回旋偏差问题的角度来提高SWM纤维束追踪技术. ...
Review of neural fiber tracking with diffusion magnetic resonance imaging
1
2022
... 在过去的十几年中,基于dMRI的大脑纤维束成像技术取得了巨大进展,dMRI的空间和角度分辨率显著提高[19].现有的大脑纤维束研究主要集中在DWM[20],为了构建完整人类连接组,一些研究者开始关注SWM纤维的连接.然而,现有的大脑纤维束成像技术都是针对于DWM来开发的,为了适应SWM的复杂纤维,一些研究者提出了专门针对于SWM纤维束连接的算法.这些算法考虑到了SWM高度弯曲、回旋偏差以及个体间皮层折叠模式高度变异性的特征,从而提高了SWM纤维束的成像质量.本节将会讨论如何从解决高度弯曲和回旋偏差问题的角度来提高SWM纤维束追踪技术. ...
扩散磁共振图像的神经纤维追踪算法研究综述
1
2022
... 在过去的十几年中,基于dMRI的大脑纤维束成像技术取得了巨大进展,dMRI的空间和角度分辨率显著提高[19].现有的大脑纤维束研究主要集中在DWM[20],为了构建完整人类连接组,一些研究者开始关注SWM纤维的连接.然而,现有的大脑纤维束成像技术都是针对于DWM来开发的,为了适应SWM的复杂纤维,一些研究者提出了专门针对于SWM纤维束连接的算法.这些算法考虑到了SWM高度弯曲、回旋偏差以及个体间皮层折叠模式高度变异性的特征,从而提高了SWM纤维束的成像质量.本节将会讨论如何从解决高度弯曲和回旋偏差问题的角度来提高SWM纤维束追踪技术. ...
Limitations and requirements of diffusion tensor fiber tracking: an assessment using simulations
1
2002
... 在dMRI纤维束成像技术的发展初期,一个主要的挑战是纤维追踪算法的高度弯曲偏差问题.这种偏差产生的原因是因为算法在追踪纤维束时,通常只在当前位置的局部纤维方向上采取一个有限的步长,这种方法被称为“一阶”方法.当纤维束的路径发生弯曲时,这种一阶方法往往会低估纤维的实际曲率,导致追踪出的路径出现偏差[21].虽然通过减小步长,即采取更小的图像体素尺寸,可以在一定程度上缓解这个问题,但这种方法并不是最理想的解决方案.更直接的方法是使用高阶积分技术,这种技术在追踪过程中能够直接考虑纤维束的曲率.然而,当需要与能够处理纤维交叉的扩散模型兼容时,这种高阶积分方法的实现就变得更加困难.简而言之,尽管高阶方法理论上能更准确地追踪纤维束,但在实际应用中,如何有效地整合这些方法仍然是一个技术难题[22]. ...
Parallel transport tractography
1
2020
... 在dMRI纤维束成像技术的发展初期,一个主要的挑战是纤维追踪算法的高度弯曲偏差问题.这种偏差产生的原因是因为算法在追踪纤维束时,通常只在当前位置的局部纤维方向上采取一个有限的步长,这种方法被称为“一阶”方法.当纤维束的路径发生弯曲时,这种一阶方法往往会低估纤维的实际曲率,导致追踪出的路径出现偏差[21].虽然通过减小步长,即采取更小的图像体素尺寸,可以在一定程度上缓解这个问题,但这种方法并不是最理想的解决方案.更直接的方法是使用高阶积分技术,这种技术在追踪过程中能够直接考虑纤维束的曲率.然而,当需要与能够处理纤维交叉的扩散模型兼容时,这种高阶积分方法的实现就变得更加困难.简而言之,尽管高阶方法理论上能更准确地追踪纤维束,但在实际应用中,如何有效地整合这些方法仍然是一个技术难题[22]. ...
Surface-based tracking of U-fibers in the superficial white matter
1
2019
... 由于SWM存在大量的高度弯曲纤维,现有的基于DWM纤维追踪算法不能重建出这种高度弯曲的纤维结构.为了解决该问题,Gahm等人[23]提出了一种基于表面的SWM纤维束追踪框架,该框架通过测量表面切空间上的角度变化,在三角网格内通过角度阈值控制路径的平滑度,并在跨三角网格时测量交叉角度,以确保追踪方向的准确性,这种方法利用了皮质表面的内在几何特性,更符合U型纤维沿着皮质表面的解剖学特性.与Gahm等人提出的确定性纤维束追踪算法不同,Nie等人[17]提出了一种新的基于表面的概率追踪框架,该框架将3D纤维取向分布(FOD)的球谐系数转换到每个三角形表面的局部坐标系中,从而将FOD投影到SWM的切空间,利用平行传输实现流线在SWM上的内在传播,根据概率抽样的纤维方向进行追踪,避免了传统基于体积追踪方法中必要的但具有挑战性的急剧转向.该算法重建了中央前回和中央后回的U型纤维,通过4个定量指标和MRtrix提供的基于体积的纤维束追踪算法以及基于表面的确定性追踪算法做对比,4个定量指标分别为重建纤维束的数量、U型纤维的连接完整性、U型纤维比率以及拓扑规律性.在重建纤维束的数量这个指标上,作者所提出的方法在所有三种方法中生成了最有效的连接,48.5%的种子点生成了有效连接.对于基于表面的确定性追踪算法,只有29.5%的种子点发展成有效连接.对于MRtrix的基于体积的纤维束追踪算法,只有6%的种子点生成有效的U型纤维连接.U型纤维的连接完整性是指将两个脑回的骨架均匀分成若干部分,统计被重建的纤维束是否在这些部分中,实验表明作者提出的算法重建出的U型纤维更完整.U型纤维比率是指两个端点之间的距离与纤维总长度的比值,较小的U型纤维比率表示成功重建有效的U型纤维,作者提出的算法相较于其他算法U型纤维比率是最低的.拓扑规律性是使用经典的多维标定方法将纤维束的起点和终点投影到二维平面,并计算它们之间的Procrustes距离,以评估重建纤维的拓扑规律性,通过计算作者提出的算法距离更短,这表明通过约束沿SWM表面的纤维追踪,可以生成形状更规则的U型纤维. ...
Confirmation of a gyral bias in diffusion MRI fiber tractography
1
2018
... 在纤维束成像技术中,回旋偏差是指追踪的纤维路径错误地终止在脑回上,而不是正确的脑沟中[24],这种偏差会降低成像结果与实际解剖结构的一致性.回旋偏差的产生有多方面的原因,其中包括大脑皮质灰质和SWM交界处轴突排列的复杂性.此外,由于MRI的空间分辨率有限,部分体积效应使得从重建的FOD中区分复杂的纤维结构变得困难. ...
Fusion in diffusion MRI for improved fibre orientation estimation: An application to the 3T and 7T data of the Human Connectome Project
1
2016
... 提高MRI图像的分辨率有助于减少这种偏差,但这种做法受到dMRI数据信噪比的限制.即使在高分辨率的dMRI数据上进行操作,现有的纤维束成像算法仍然存在偏差,与组织学染色揭示的真实纤维投影相比,脑回冠的纤维路径密度通常比脑沟岸更大[25].此外,大脑皮质的复杂折叠和卷曲也给长程纤维连接的准确重建带来了挑战.尽管提高分辨率可以改善成像质量,但要完全消除回旋偏差,还需要开发一种新颖的成像技术. ...
Improved delineation of short cortical association fibers and gray/white matter boundary using whole-brain three-dimensional diffusion tensor imaging at submillimeter spatial resolution
1
2014
... SWM纤维在低空间分辨率下很难被清晰识别,为了提高空间分辨率,改善图像质量,Song等人[26]使用了0.85毫米各向同性空间分辨率的DTI技术,该成像技术显著提高了短关联纤维(U型纤维)的追踪效果.该工作首次通过提高DTI的空间分辨率来进行短关联纤维的追踪,但是该技术在活体大脑连接成像中尚未常规实现.由于SWM区域存在回旋偏差,为了减少回旋偏差,一些研究者做出了巨大的贡献.St-Onge等人[27]提出了一种基于表面流的增强纤维束追踪算法.表面流是平均曲率流的改进,通过基于从T1加权图像提取的皮质表面几何形状(顶点、法线、面积和曲率)和改进表面播种和终止策略,以提高SWM纤维束成像的分辨率和精度.该方法减少了回旋偏差、长度偏差和假阳性流线的数量. ...
Surface-enhanced tractography (SET)
1
2018
... SWM纤维在低空间分辨率下很难被清晰识别,为了提高空间分辨率,改善图像质量,Song等人[26]使用了0.85毫米各向同性空间分辨率的DTI技术,该成像技术显著提高了短关联纤维(U型纤维)的追踪效果.该工作首次通过提高DTI的空间分辨率来进行短关联纤维的追踪,但是该技术在活体大脑连接成像中尚未常规实现.由于SWM区域存在回旋偏差,为了减少回旋偏差,一些研究者做出了巨大的贡献.St-Onge等人[27]提出了一种基于表面流的增强纤维束追踪算法.表面流是平均曲率流的改进,通过基于从T1加权图像提取的皮质表面几何形状(顶点、法线、面积和曲率)和改进表面播种和终止策略,以提高SWM纤维束成像的分辨率和精度.该方法减少了回旋偏差、长度偏差和假阳性流线的数量. ...
Improved tractography using asymmetric fibre orientation distributions
1
2017
... 近年来,一些研究者使用非对称纤维方向分布来解决SWM回旋偏差的问题,Bastiani等人[28]提出了一种改进的纤维束成像技术,该技术基于非对称纤维方向分布,能够通过分析周围体素的空间信息来减少成像过程中的回旋偏差.这种方法可以更精确地推断出体素内部的纤维结构,尤其是对于那些结构复杂的纤维,如急剧弯曲和扇形分布纤维.通过与高分辨率组织学数据的比较,验证了该方法能够可靠地估计复杂的纤维模式.同样基于非对称纤维方向分布方法,Wu等人[29]提出了一种基于全局估计框架的非对称纤维方向分布函数的方法,以减轻大脑皮层纤维追踪中的回旋偏差.通过多组织全局估计框架计算非对称纤维方向分布函数,该方法能够使纤维流线在脑回叶片的灰质-白质边界处更锐利地转向皮层灰质,从而提高了纤维追踪的准确性,并在不同场强(3 T和7 T)的磁共振成像数据之间提供了高度一致的结果.这项工作解决了现有纤维追踪算法中存在的回旋偏差问题,即纤维流线主要终止于脑回顶部而非脑沟边缘,导致连接分析的严重偏差. ...
Mitigating gyral bias in cortical tractography via asymmetric fiber orientation distributions
1
2020
... 近年来,一些研究者使用非对称纤维方向分布来解决SWM回旋偏差的问题,Bastiani等人[28]提出了一种改进的纤维束成像技术,该技术基于非对称纤维方向分布,能够通过分析周围体素的空间信息来减少成像过程中的回旋偏差.这种方法可以更精确地推断出体素内部的纤维结构,尤其是对于那些结构复杂的纤维,如急剧弯曲和扇形分布纤维.通过与高分辨率组织学数据的比较,验证了该方法能够可靠地估计复杂的纤维模式.同样基于非对称纤维方向分布方法,Wu等人[29]提出了一种基于全局估计框架的非对称纤维方向分布函数的方法,以减轻大脑皮层纤维追踪中的回旋偏差.通过多组织全局估计框架计算非对称纤维方向分布函数,该方法能够使纤维流线在脑回叶片的灰质-白质边界处更锐利地转向皮层灰质,从而提高了纤维追踪的准确性,并在不同场强(3 T和7 T)的磁共振成像数据之间提供了高度一致的结果.这项工作解决了现有纤维追踪算法中存在的回旋偏差问题,即纤维流线主要终止于脑回顶部而非脑沟边缘,导致连接分析的严重偏差. ...
Surface-based tracking for short association fibre tractography
1
2022
... Shastin等人[30]采用了表面种子点选择策略,提出了一种全脑短关联纤维算法,该算法加入了纤维流线的过滤策略,包括灰质-灰质滤波、半球-半球滤波和灰质-白质-灰质滤波.所提出的算法能够产生更长的流线并且倾向于连接脑回,同样减少了回旋偏差.与上述减少回旋偏差的方法不同,Cottaar等人[31]将脑白质在脑回叶片中的建模作为一个连续的无散度向量场,以减少在白质和皮层灰质边界处的回旋偏差,该算法同时考虑了纤维密度和方向,鼓励沿皮层白/灰质边界的解剖学合理流线密度分布,同时保持与扩散MRI估计的纤维方向一致. ...
Modelling white matter in gyral blades as a continuous vector field
1
2021
... Shastin等人[30]采用了表面种子点选择策略,提出了一种全脑短关联纤维算法,该算法加入了纤维流线的过滤策略,包括灰质-灰质滤波、半球-半球滤波和灰质-白质-灰质滤波.所提出的算法能够产生更长的流线并且倾向于连接脑回,同样减少了回旋偏差.与上述减少回旋偏差的方法不同,Cottaar等人[31]将脑白质在脑回叶片中的建模作为一个连续的无散度向量场,以减少在白质和皮层灰质边界处的回旋偏差,该算法同时考虑了纤维密度和方向,鼓励沿皮层白/灰质边界的解剖学合理流线密度分布,同时保持与扩散MRI估计的纤维方向一致. ...
Tractostorm: The what, why, and how of tractography dissection reproducibility
1
2020
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
Fiber tract-based atlas of human white matter anatomy
2
2004
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Short frontal lobe connections of the human brain
2
2012
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Atlasing the frontal lobe connections and their variability due to age and education: a spherical deconvolution tractography study
2
2016
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
White matter connections of the inferior parietal lobule: a study of surgical anatomy
2
2017
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Short parietal lobe connections of the human and monkey brain
2
2017
... 传统的SWM纤维束分割方法依赖于手动纤维流线选择,也称为虚拟解剖,解剖学专家手动在大脑中绘制的ROI交互地选择纤维束流线[32].通常,包含ROI选择在皮质和皮质下,以定义流线应终止的位置,或者在白质中选择ROI,以定义流线应经过的位置,排除ROI选择的其他区域,以排除不需要的纤维流线.手动纤维流线选择被认为是纤维束追踪中描绘解剖纤维束的黄金标准,并已广泛用于验证其他解剖纤维束识别技术.Catani等人[7]是最早通过手动放置ROI来研究SWM纤维束的连接,该研究通过放置两个ROI证明了枕颞区存在U型纤维,且连接大脑邻近的脑回.Wakana等人[33]研究了大脑中的长纤维和短关联纤维.研究发现,额叶区和枕叶区存在短关联纤维,这些短关联纤维可能是额叶上纵束的一部分.Catani等人[34]在他第一个工作的基础上使用两个感兴趣区域在TrackVis中进行虚拟解剖,以隔离单一的纤维束,最后重建出额叶和顶叶的短关联纤维.Wu等人[14]利用DSI软件中放置排除的纤维流线区域来提取短关联纤维连接,该研究集中在颞叶、顶叶和枕叶,最终确定了三个束:上纵束后段,连接颞中回和下回的后部以及角回和边缘上回;垂直枕束,连接下顶叶和下颞叶和枕叶;以及一种新型的颞顶连接,将颞下回、颞中回和梭状回以及枕下叶与顶上叶相互连接.Rojkova等人[35]通过47名受试者的HARDI数据构建统计图谱,并研究他们在年龄和教育方面的变异性.该图谱追踪出30个额叶短U型束,为临床提供了有价值的研究.Burks等人[36]通过手动定义ROI来启动纤维追踪,研究顶下小叶的纤维束连接关系,研究发现短关联纤维连接上脑回和角回,并将这两个回连接到顶上小叶.Catani等人[37]同时研究人类和猴子顶叶的短关联纤维连接,最后通过实验在顶叶内侧和外侧都发现了短的U型纤维. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Pyramid-shape crossings and intercrossing fibers are key elements for construction of the neural network in the superficial white matter of the human cerebrum
2
2020
... 尽管这些研究都能够获得非常明确的SWM纤维束,为大脑的短连接提供了新的见解,但是这些研究仅限于大脑的特定区域.Shinohara等人[38]通过DSI软件追踪纤维束成像成功地揭示了一种新型的U型纤维,这些U型纤维与在沟底脑回间U型纤维不同,它隐藏在脑回白质脊中并沿着脑回内U型纤维延伸.脑回内和脑回间的U型纤维从不同方向会聚到白质脊的交界区域,形成新的解剖结构,即“金字塔形交叉”. 形成金字塔形交叉的U型纤维也为交叉点之间的信息传递提供了路线.这项研究发现在每个外侧皮质表面平均有97个金字塔形交叉,它们可能成为大脑皮层分割的新解剖标志. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Human brain white matter atlas: identification and assignment of common anatomical structures in superficial white matter
2
2008
... 尽管手动选择ROI是描绘纤维束的黄金标准,但是手动选择ROI非常耗时且需要具备专业临床解剖知识的专家,这就造成临床和专家劳动力成本较高.目前有很多研究者抛弃了手动ROI选择,改为自动ROI的选择.大多数基于自动ROI的方法利用大脑ROI图谱并使用图像配准来自动将图谱标准空间中的ROI与受试者空间对齐.Oishi等人[39]是第一个通过自动选择ROI来进行SWM纤维束分割的研究者,他们使用了81名健康受试者的DTI数据,创建了基于群体平均的白质解剖图谱,识别了9个常见的脑回刀片状的解剖区域,并将它们进一步划分为21个子区域,使用定义的SWM区域作为ROI,重建感兴趣的SWM纤维束,并在10名未包含在该图谱数据集中的正常受试者的数据上重复实验,以验证所识别的纤维束的可重复性,结果表明在健康人群中可重复识别出连接相邻回的四条短关联纤维束. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Atlas-guided tract reconstruction for automated and comprehensive examination of the white matter anatomy
2
2010
... Zhang等人[40]提出一种自动化方法,利用存储在DTI基础脑图谱中的关于纤维束轨迹的解剖知识,以非线性配准方法配准到单个受试者的DTI数据,从而自动化地重建大量白质纤维束,尤其是重建出29个短关联纤维束.Pardo等人[41]使用同样的方法,将30个受试者的HARDI数据非线性配准到JHU MNI SS WMPM TypeII图谱,根据每个束连接的两个皮层区域,分割40个短关联白质纤维束,并研究获得的SWM纤维束的可变性.Ouyang等人[42]的工作与其他研究者略有区别,他们将21名受试者的DTI数据的大脑皮层分割为每个半球34个脑回,连接两个脑回的纤维被提取到整个大脑,并仅将那些连接相邻脑回的纤维归类为短关联纤维,并提出了皮层连接成熟指数(cortical connectivity maturation index,CCMI),CCMI通过区分长纤维和短关联纤维,量化了短关联纤维的数量,这在以往的研究中往往被忽视.为了研究U型纤维的可重复性和可靠性,Movahedian等人[43]使用具有300 mT/m最大梯度幅度的先进MRI扫描仪,实现亚毫米级分辨率的扩散MRI,对视觉皮层V1和V2区域之间的解剖连接进行U型纤维追踪,通过在不同参与者和独立复制组中重复测量,展示了所提出U型纤维连接映射方法的高重复性和可靠性. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Study of the variability of short association bundles on a HARDI database
2
2013
... Zhang等人[40]提出一种自动化方法,利用存储在DTI基础脑图谱中的关于纤维束轨迹的解剖知识,以非线性配准方法配准到单个受试者的DTI数据,从而自动化地重建大量白质纤维束,尤其是重建出29个短关联纤维束.Pardo等人[41]使用同样的方法,将30个受试者的HARDI数据非线性配准到JHU MNI SS WMPM TypeII图谱,根据每个束连接的两个皮层区域,分割40个短关联白质纤维束,并研究获得的SWM纤维束的可变性.Ouyang等人[42]的工作与其他研究者略有区别,他们将21名受试者的DTI数据的大脑皮层分割为每个半球34个脑回,连接两个脑回的纤维被提取到整个大脑,并仅将那些连接相邻脑回的纤维归类为短关联纤维,并提出了皮层连接成熟指数(cortical connectivity maturation index,CCMI),CCMI通过区分长纤维和短关联纤维,量化了短关联纤维的数量,这在以往的研究中往往被忽视.为了研究U型纤维的可重复性和可靠性,Movahedian等人[43]使用具有300 mT/m最大梯度幅度的先进MRI扫描仪,实现亚毫米级分辨率的扩散MRI,对视觉皮层V1和V2区域之间的解剖连接进行U型纤维追踪,通过在不同参与者和独立复制组中重复测量,展示了所提出U型纤维连接映射方法的高重复性和可靠性. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Global and regional cortical connectivity maturation index (CCMI) of developmental human brain with quantification of short-range association tracts
2
2016
... Zhang等人[40]提出一种自动化方法,利用存储在DTI基础脑图谱中的关于纤维束轨迹的解剖知识,以非线性配准方法配准到单个受试者的DTI数据,从而自动化地重建大量白质纤维束,尤其是重建出29个短关联纤维束.Pardo等人[41]使用同样的方法,将30个受试者的HARDI数据非线性配准到JHU MNI SS WMPM TypeII图谱,根据每个束连接的两个皮层区域,分割40个短关联白质纤维束,并研究获得的SWM纤维束的可变性.Ouyang等人[42]的工作与其他研究者略有区别,他们将21名受试者的DTI数据的大脑皮层分割为每个半球34个脑回,连接两个脑回的纤维被提取到整个大脑,并仅将那些连接相邻脑回的纤维归类为短关联纤维,并提出了皮层连接成熟指数(cortical connectivity maturation index,CCMI),CCMI通过区分长纤维和短关联纤维,量化了短关联纤维的数量,这在以往的研究中往往被忽视.为了研究U型纤维的可重复性和可靠性,Movahedian等人[43]使用具有300 mT/m最大梯度幅度的先进MRI扫描仪,实现亚毫米级分辨率的扩散MRI,对视觉皮层V1和V2区域之间的解剖连接进行U型纤维追踪,通过在不同参与者和独立复制组中重复测量,展示了所提出U型纤维连接映射方法的高重复性和可靠性. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Mapping short association fibers in the early cortical visual processing stream using in vivo diffusion tractography
2
2020
... Zhang等人[40]提出一种自动化方法,利用存储在DTI基础脑图谱中的关于纤维束轨迹的解剖知识,以非线性配准方法配准到单个受试者的DTI数据,从而自动化地重建大量白质纤维束,尤其是重建出29个短关联纤维束.Pardo等人[41]使用同样的方法,将30个受试者的HARDI数据非线性配准到JHU MNI SS WMPM TypeII图谱,根据每个束连接的两个皮层区域,分割40个短关联白质纤维束,并研究获得的SWM纤维束的可变性.Ouyang等人[42]的工作与其他研究者略有区别,他们将21名受试者的DTI数据的大脑皮层分割为每个半球34个脑回,连接两个脑回的纤维被提取到整个大脑,并仅将那些连接相邻脑回的纤维归类为短关联纤维,并提出了皮层连接成熟指数(cortical connectivity maturation index,CCMI),CCMI通过区分长纤维和短关联纤维,量化了短关联纤维的数量,这在以往的研究中往往被忽视.为了研究U型纤维的可重复性和可靠性,Movahedian等人[43]使用具有300 mT/m最大梯度幅度的先进MRI扫描仪,实现亚毫米级分辨率的扩散MRI,对视觉皮层V1和V2区域之间的解剖连接进行U型纤维追踪,通过在不同参与者和独立复制组中重复测量,展示了所提出U型纤维连接映射方法的高重复性和可靠性. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
White matter connections of the supplementary motor area in humans
2
2014
... 基于自动选择ROI的SWM纤维束分割方法依赖于标准图谱的质量以及配准过程,解剖区域在不同受试者和病理之间存在差异,而且在定义好的ROI区域会捕获大量的异常纤维流线.为了解决上述问题,一些研究者提出了半自动ROI选择算法,通过标准图谱自动选择ROI区域,并使用手动ROI放置排除一些异常纤维流线.Vergani等人[44]通过非线性变换,将每个受试者配准到蒙特利尔神经研究所(montreal neurological institute,MNI)标准空间,选择辅助运动区为研究对象,研究其短关联纤维的连接,并通过手动放置ROI排除区域,以排除剩余的虚假流线或解剖学上不一致的流线.实验发现U型纤维在中央前沟和扣带沟中存在,中央前回和辅助运动区相连接,辅助运动区和扣带回相连接.与上述半自动选择ROI的工作不同,Magro等人[45]通过临床专家手动绘制2D ROI区域,然后自动扩充到3D脑回叶片,使用纤维束成像研究中央前回和中央后回的短关联纤维连接. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Characterization of short white matter fiber bundles in the central area from diffusion tensor MRI
2
2012
... 基于自动选择ROI的SWM纤维束分割方法依赖于标准图谱的质量以及配准过程,解剖区域在不同受试者和病理之间存在差异,而且在定义好的ROI区域会捕获大量的异常纤维流线.为了解决上述问题,一些研究者提出了半自动ROI选择算法,通过标准图谱自动选择ROI区域,并使用手动ROI放置排除一些异常纤维流线.Vergani等人[44]通过非线性变换,将每个受试者配准到蒙特利尔神经研究所(montreal neurological institute,MNI)标准空间,选择辅助运动区为研究对象,研究其短关联纤维的连接,并通过手动放置ROI排除区域,以排除剩余的虚假流线或解剖学上不一致的流线.实验发现U型纤维在中央前沟和扣带沟中存在,中央前回和辅助运动区相连接,辅助运动区和扣带回相连接.与上述半自动选择ROI的工作不同,Magro等人[45]通过临床专家手动绘制2D ROI区域,然后自动扩充到3D脑回叶片,使用纤维束成像研究中央前回和中央后回的短关联纤维连接. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Functional clustering of whole brain white matter fibers
1
2020
... 基于流线标记的分割方法是指为每个单独的流线分配解剖标签.通常,流线标记是通过计算每条流线与参考区域分割中标记流线的几何距离来分配流线标签完成的[46].流线标记的分割方法可以大致分为基于几何距离的流线标记、基于聚类的流线标记和基于深度学习的流线标记. ...
Disentangling the variability of the superficial white matter organization using regional-tractogram-based population stratification
2
2022
... 基于几何距离的流线标记方法是指为每条流线进行标记来分配解剖标签.常用的几何距离是欧几里得距离和流线长度,由于SWM纤维束个体间差异较大,单纯的欧几里得距离和流线长度不能满足SWM纤维束的分割.Guevara等人[47]考虑了纤维的空间方向,引入了一种新的纤维距离度量,以更精细地推断短束周围的纤维图谱,并提出了一种子图集距离度量方法,用于量化两个不同个体的纤维集合之间的相似性,最后通过流形学习方法根据纤维束的几何距离,在多维空间中将人群划分为具有相似区域纤维组织的群体,在中央沟和颞上沟解开了浅层白质束的组织变异性.Vindas等人[48]提出了一种名为GeoLab的分割方法,该方法使用6个参数描述纤维束的几何特征,包括流线长度、到束质心的欧几里得距离、平面间角度、方向间角度、形状角和最小化的最大欧几里得距离,这些几何特征能够从单个受试者中高效地分割出数百个SWM束. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
GeoLab: geometry-based tractography parcellation of superficial white matter
2
2023
... 基于几何距离的流线标记方法是指为每条流线进行标记来分配解剖标签.常用的几何距离是欧几里得距离和流线长度,由于SWM纤维束个体间差异较大,单纯的欧几里得距离和流线长度不能满足SWM纤维束的分割.Guevara等人[47]考虑了纤维的空间方向,引入了一种新的纤维距离度量,以更精细地推断短束周围的纤维图谱,并提出了一种子图集距离度量方法,用于量化两个不同个体的纤维集合之间的相似性,最后通过流形学习方法根据纤维束的几何距离,在多维空间中将人群划分为具有相似区域纤维组织的群体,在中央沟和颞上沟解开了浅层白质束的组织变异性.Vindas等人[48]提出了一种名为GeoLab的分割方法,该方法使用6个参数描述纤维束的几何特征,包括流线长度、到束质心的欧几里得距离、平面间角度、方向间角度、形状角和最小化的最大欧几里得距离,这些几何特征能够从单个受试者中高效地分割出数百个SWM束. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Automatic fiber bundle segmentation in massive tractography datasets using a multi-subject bundle atlas
4
2012
... 为了减少每个流线的标记量,许多流线标记方法首先将流线分组为簇,然后为每个簇分配解剖标签,这种方法被称为聚类方法.Guevara等人[49]是第一个在纤维束聚类中涉及SWM工作的,他们的工作通过层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分组,提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM图谱还包含每个半球的47个SWM束.该研究主要对DWM进行聚类,并未对SWM进行针对性的研究.Zhang等人[13]的研究是第一个仅针对于SWM纤维束的研究工作,他们同样使用欧几里得距离进行纤维聚类,从DTI、HARDI、DSI数据中识别和表征U型纤维,证明了大多数U型纤维通过沿脑沟来连接相邻的脑回.Román等人[50]提出的方法是Guevara等人提出方法的改进,该方法专门针对短关联纤维进行聚类,并且只选择35~85 mm的短纤维,同时该方法还使用了Desikan-Killiany FreeSurfer皮质分区[51]自动命名生成SWM纤维束,最终通过74名健康受试者的高质量HARDI数据生成了一个包含左半球44个、右半球49个以及两个半球共有的33个SWM纤维束的图谱. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
... Guevara等人[49]首次在构建DWM脑图谱时涉及SWM脑图谱,他们使用凝聚平均链接层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分割,最终生成左半球47个SWM束的图谱,然后根据连接区域的功能手动标记这些图谱.Román等人[50]同样使用分层聚类的方法和欧几里得距离测量对整个大脑纤维束进行分割以创建图谱,与Guevara等人的工作不同的是,该研究专注于SWM,获得的SWM图谱中左半球有44个束,右半球有49个束,其中33个束分布在两个半球.Guevara等人[56]在聚类方法的基础上引入自动选取ROI算法,通过这种混合的方法以确定跨受试者的可重复、定义明确的束,最终创建了一个包含100个可重复束的SWM图谱,其中35个是两个半球共有的.Zhang等人[52]为了创建一个精细的全脑纤维束图谱,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,最终创建出一个包括58个DWM纤维束和198个短程和中程的SWM纤维束图谱,并且通过不同类型的受试者进行了验证,证明了该图谱的可重复性.Román等人[57]在先前工作的基础上,引入自动选取ROI的方法,与聚类方法相结合,最终创建的SWM图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI对,141束连接相同ROI的部分.5种SWM纤维束图谱的构建方法详细对比如表2所示. ...
... Comparison of SWM fiber tract atlas construction methods
Table 2 | 第一作者 | 图谱构成 | 构建数据 | 测试数据 | 纤维束追踪方法 | 概率性/确定性追踪 | 构建方法 |
Guevara[49]
| 36个DWM束,94个SWM束 | 12 NMR
| 20 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Román[50]
| 93个SWM束
| 74 CONNECT/Archi
| 78 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Zhang[52]
| 58 DWM束,198个SWM束
| 100名健康受试者
| 584名患有多
种健康状况的
受试者 | 双张量无迹
卡尔曼滤波
| 确定性
| 谱聚类
|
Guevara[56]
| 100个SWM束
| 79 CONNECT/Archi
| 26 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 自动ROI选择+层次聚类 |
Román[57]
| 525个SWM束
| 100 HCP
| 79 HARDI
| CSD+iFOD2
| 概率性
| 自动ROI选择+FFClust聚类+层次聚类 |
4 总结与展望本文对基于dMRI的SWM纤维束研究进行了广泛的调研,从SWM纤维束追踪技术,SWM纤维束分割方法,SWM纤维束脑图谱的构建等方面,总结了SWM纤维束成像与分析方面的研究进展.通过全面调研发现SWM纤维束成像方法的研究侧重点已经发生改变,从原来使用DWM纤维束成像方法逐渐变为针对于SWM的特点设计新颖的追踪方法.SWM纤维束分割的方法不再是手动选取ROI进行分割,而是逐渐变为自动选取ROI或者使用无监督聚类的方法进行SWM纤维束分割,近年来深度学习的方法也逐渐应用于SWM纤维束的分割.在此基础上,SWM脑图谱构建也更加细化,各ROI区域的连接被深入研究,分割出的簇越来越多. ...
Clustering of whole-brain white matter short association bundles using HARDI data
4
2017
... 为了减少每个流线的标记量,许多流线标记方法首先将流线分组为簇,然后为每个簇分配解剖标签,这种方法被称为聚类方法.Guevara等人[49]是第一个在纤维束聚类中涉及SWM工作的,他们的工作通过层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分组,提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM图谱还包含每个半球的47个SWM束.该研究主要对DWM进行聚类,并未对SWM进行针对性的研究.Zhang等人[13]的研究是第一个仅针对于SWM纤维束的研究工作,他们同样使用欧几里得距离进行纤维聚类,从DTI、HARDI、DSI数据中识别和表征U型纤维,证明了大多数U型纤维通过沿脑沟来连接相邻的脑回.Román等人[50]提出的方法是Guevara等人提出方法的改进,该方法专门针对短关联纤维进行聚类,并且只选择35~85 mm的短纤维,同时该方法还使用了Desikan-Killiany FreeSurfer皮质分区[51]自动命名生成SWM纤维束,最终通过74名健康受试者的高质量HARDI数据生成了一个包含左半球44个、右半球49个以及两个半球共有的33个SWM纤维束的图谱. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
... Guevara等人[49]首次在构建DWM脑图谱时涉及SWM脑图谱,他们使用凝聚平均链接层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分割,最终生成左半球47个SWM束的图谱,然后根据连接区域的功能手动标记这些图谱.Román等人[50]同样使用分层聚类的方法和欧几里得距离测量对整个大脑纤维束进行分割以创建图谱,与Guevara等人的工作不同的是,该研究专注于SWM,获得的SWM图谱中左半球有44个束,右半球有49个束,其中33个束分布在两个半球.Guevara等人[56]在聚类方法的基础上引入自动选取ROI算法,通过这种混合的方法以确定跨受试者的可重复、定义明确的束,最终创建了一个包含100个可重复束的SWM图谱,其中35个是两个半球共有的.Zhang等人[52]为了创建一个精细的全脑纤维束图谱,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,最终创建出一个包括58个DWM纤维束和198个短程和中程的SWM纤维束图谱,并且通过不同类型的受试者进行了验证,证明了该图谱的可重复性.Román等人[57]在先前工作的基础上,引入自动选取ROI的方法,与聚类方法相结合,最终创建的SWM图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI对,141束连接相同ROI的部分.5种SWM纤维束图谱的构建方法详细对比如表2所示. ...
... Comparison of SWM fiber tract atlas construction methods
Table 2 | 第一作者 | 图谱构成 | 构建数据 | 测试数据 | 纤维束追踪方法 | 概率性/确定性追踪 | 构建方法 |
Guevara[49]
| 36个DWM束,94个SWM束 | 12 NMR
| 20 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Román[50]
| 93个SWM束
| 74 CONNECT/Archi
| 78 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Zhang[52]
| 58 DWM束,198个SWM束
| 100名健康受试者
| 584名患有多
种健康状况的
受试者 | 双张量无迹
卡尔曼滤波
| 确定性
| 谱聚类
|
Guevara[56]
| 100个SWM束
| 79 CONNECT/Archi
| 26 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 自动ROI选择+层次聚类 |
Román[57]
| 525个SWM束
| 100 HCP
| 79 HARDI
| CSD+iFOD2
| 概率性
| 自动ROI选择+FFClust聚类+层次聚类 |
4 总结与展望本文对基于dMRI的SWM纤维束研究进行了广泛的调研,从SWM纤维束追踪技术,SWM纤维束分割方法,SWM纤维束脑图谱的构建等方面,总结了SWM纤维束成像与分析方面的研究进展.通过全面调研发现SWM纤维束成像方法的研究侧重点已经发生改变,从原来使用DWM纤维束成像方法逐渐变为针对于SWM的特点设计新颖的追踪方法.SWM纤维束分割的方法不再是手动选取ROI进行分割,而是逐渐变为自动选取ROI或者使用无监督聚类的方法进行SWM纤维束分割,近年来深度学习的方法也逐渐应用于SWM纤维束的分割.在此基础上,SWM脑图谱构建也更加细化,各ROI区域的连接被深入研究,分割出的簇越来越多. ...
An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest
1
2006
... 为了减少每个流线的标记量,许多流线标记方法首先将流线分组为簇,然后为每个簇分配解剖标签,这种方法被称为聚类方法.Guevara等人[49]是第一个在纤维束聚类中涉及SWM工作的,他们的工作通过层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分组,提出了一种基于多受试者的纤维束图谱,该图谱中除了DWM图谱还包含每个半球的47个SWM束.该研究主要对DWM进行聚类,并未对SWM进行针对性的研究.Zhang等人[13]的研究是第一个仅针对于SWM纤维束的研究工作,他们同样使用欧几里得距离进行纤维聚类,从DTI、HARDI、DSI数据中识别和表征U型纤维,证明了大多数U型纤维通过沿脑沟来连接相邻的脑回.Román等人[50]提出的方法是Guevara等人提出方法的改进,该方法专门针对短关联纤维进行聚类,并且只选择35~85 mm的短纤维,同时该方法还使用了Desikan-Killiany FreeSurfer皮质分区[51]自动命名生成SWM纤维束,最终通过74名健康受试者的高质量HARDI数据生成了一个包含左半球44个、右半球49个以及两个半球共有的33个SWM纤维束的图谱. ...
An anatomically curated fiber clustering white matter atlas for consistent white matter tract parcellation across the lifespan
4
2018
... 为了创建一个全脑的纤维束图谱,Zhang等人[52]利用来自100名受试者的信息,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,创建了一个基于纤维的白质图谱,包括58个DWM纤维束和198个短程和中程的SWM纤维束.该图谱在多个受试者中被证明是稳定的,在所有受试者中都检测到了图谱中注释的99%以上的纤维束.Pron等人[53]只研究了中央沟的U型纤维,通过k-medoids聚类算法在左脑中央沟进行聚类,分割出五条U型纤维.基于这个工作,Pron等人[54]在后续的研究中又完善了表征中央沟U型纤维的工作,他们使用基于密度的噪声应用空间聚类(DBSCAN)算法对连接空间中的群体连接性轮廓进行聚类,以提取纤维束,在大脑左右半球的中央沟周围各提取出五条U型纤维,并证明了手功能区的U型纤维与用手习惯的显著关系. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
... Guevara等人[49]首次在构建DWM脑图谱时涉及SWM脑图谱,他们使用凝聚平均链接层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分割,最终生成左半球47个SWM束的图谱,然后根据连接区域的功能手动标记这些图谱.Román等人[50]同样使用分层聚类的方法和欧几里得距离测量对整个大脑纤维束进行分割以创建图谱,与Guevara等人的工作不同的是,该研究专注于SWM,获得的SWM图谱中左半球有44个束,右半球有49个束,其中33个束分布在两个半球.Guevara等人[56]在聚类方法的基础上引入自动选取ROI算法,通过这种混合的方法以确定跨受试者的可重复、定义明确的束,最终创建了一个包含100个可重复束的SWM图谱,其中35个是两个半球共有的.Zhang等人[52]为了创建一个精细的全脑纤维束图谱,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,最终创建出一个包括58个DWM纤维束和198个短程和中程的SWM纤维束图谱,并且通过不同类型的受试者进行了验证,证明了该图谱的可重复性.Román等人[57]在先前工作的基础上,引入自动选取ROI的方法,与聚类方法相结合,最终创建的SWM图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI对,141束连接相同ROI的部分.5种SWM纤维束图谱的构建方法详细对比如表2所示. ...
... Comparison of SWM fiber tract atlas construction methods
Table 2 | 第一作者 | 图谱构成 | 构建数据 | 测试数据 | 纤维束追踪方法 | 概率性/确定性追踪 | 构建方法 |
Guevara[49]
| 36个DWM束,94个SWM束 | 12 NMR
| 20 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Román[50]
| 93个SWM束
| 74 CONNECT/Archi
| 78 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Zhang[52]
| 58 DWM束,198个SWM束
| 100名健康受试者
| 584名患有多
种健康状况的
受试者 | 双张量无迹
卡尔曼滤波
| 确定性
| 谱聚类
|
Guevara[56]
| 100个SWM束
| 79 CONNECT/Archi
| 26 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 自动ROI选择+层次聚类 |
Román[57]
| 525个SWM束
| 100 HCP
| 79 HARDI
| CSD+iFOD2
| 概率性
| 自动ROI选择+FFClust聚类+层次聚类 |
4 总结与展望本文对基于dMRI的SWM纤维束研究进行了广泛的调研,从SWM纤维束追踪技术,SWM纤维束分割方法,SWM纤维束脑图谱的构建等方面,总结了SWM纤维束成像与分析方面的研究进展.通过全面调研发现SWM纤维束成像方法的研究侧重点已经发生改变,从原来使用DWM纤维束成像方法逐渐变为针对于SWM的特点设计新颖的追踪方法.SWM纤维束分割的方法不再是手动选取ROI进行分割,而是逐渐变为自动选取ROI或者使用无监督聚类的方法进行SWM纤维束分割,近年来深度学习的方法也逐渐应用于SWM纤维束的分割.在此基础上,SWM脑图谱构建也更加细化,各ROI区域的连接被深入研究,分割出的簇越来越多. ...
Dense and structured representations of U-shape fiber connectivity in the central sulcus
2
2018
... 为了创建一个全脑的纤维束图谱,Zhang等人[52]利用来自100名受试者的信息,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,创建了一个基于纤维的白质图谱,包括58个DWM纤维束和198个短程和中程的SWM纤维束.该图谱在多个受试者中被证明是稳定的,在所有受试者中都检测到了图谱中注释的99%以上的纤维束.Pron等人[53]只研究了中央沟的U型纤维,通过k-medoids聚类算法在左脑中央沟进行聚类,分割出五条U型纤维.基于这个工作,Pron等人[54]在后续的研究中又完善了表征中央沟U型纤维的工作,他们使用基于密度的噪声应用空间聚类(DBSCAN)算法对连接空间中的群体连接性轮廓进行聚类,以提取纤维束,在大脑左右半球的中央沟周围各提取出五条U型纤维,并证明了手功能区的U型纤维与用手习惯的显著关系. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
U-shape short-range extrinsic connectivity organisation around the human central sulcus
2
2021
... 为了创建一个全脑的纤维束图谱,Zhang等人[52]利用来自100名受试者的信息,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,创建了一个基于纤维的白质图谱,包括58个DWM纤维束和198个短程和中程的SWM纤维束.该图谱在多个受试者中被证明是稳定的,在所有受试者中都检测到了图谱中注释的99%以上的纤维束.Pron等人[53]只研究了中央沟的U型纤维,通过k-medoids聚类算法在左脑中央沟进行聚类,分割出五条U型纤维.基于这个工作,Pron等人[54]在后续的研究中又完善了表征中央沟U型纤维的工作,他们使用基于密度的噪声应用空间聚类(DBSCAN)算法对连接空间中的群体连接性轮廓进行聚类,以提取纤维束,在大脑左右半球的中央沟周围各提取出五条U型纤维,并证明了手功能区的U型纤维与用手习惯的显著关系. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Superficial white matter analysis: An efficient point-cloud-based deep learning framework with supervised contrastive learning for consistent tractography parcellation across populations and dMRI acquisitions
2
2023
... 基于深度学习的流线标记分割方法是根据已有的纤维束分割数据训练模型,并预测新受试者中每个流线的解剖标签.现有的基于深度学习方法的纤维束分割方法大多数都是针对于DWM纤维束进行分割,很少有针对于SWM纤维束进行分割的方法.Xue等人[55]提出了一个名为Superficial White Matter Analysis(SupWMA)的新型两阶段深度学习框架,用于从全脑纤维束中高效且一致地分割198个SWM簇.该深度学习框架适应了基于点云的网络到SWM分割任务,在第二阶段使用监督对比学习,以增强SWM纤维束的特征学习,通过数据增强技术获得SWM合理纤维束和异常值之间更具区分性的表示.该研究是第一个通过深度学习来进行SWM纤维束分割的工作.Zhang等人[15]提出了一种新的基于解剖学引导的SWM纤维束分割框架,该框架引入了一种独特的纤维解剖学描述符,称为FiberAnatMap,它结合了个体和群体水平的解剖学特征,利用基于点云深度学习网络,将纤维的空间坐标和FiberAnatMap作为输入,提高了分割的准确性,用于改善深度学习网络对SWM纤维束的分割性能. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
Reproducibility of superficial white matter tracts using diffusion-weighted imaging tractography
4
2017
... 基于ROI选择和聚类相结合的方法是指在提前定义好的ROI区域进行聚类.ROI选择可以提取和标记有意义的纤维束区域,并加快聚类速度,聚类可以有效过滤异常值,将两者相结合可以分割出有解剖学意义的纤维束.基于这种思想,一些混合的SWM纤维束分割方法被提出,Guevara等人[56]通过基于ROI选择和聚类的算法创建了全脑的SWM纤维束图谱,首先根据Desikan-Killiany图谱对每个受试者的T1加权MRI图像进行皮层分割,接着利用皮层分割结果,识别连接不同皮层ROI的纤维,最后通过受试者内聚类和受试者间聚类创建出最终的SWM纤维束图谱.Román等人[57]同样用这种方法进行SWM纤维束图谱的构建,与上述工作不同的是该研究首先进行纤维束聚类,再根据ROI分组.具体做法是首先在每个受试者内部,使用快速纤维聚类(fast fiber clustering,FFClust)算法对纤维进行聚类,以形成紧凑的纤维簇,每组纤维代表一个区域的纤维簇,并生成每个簇的质心.其次,基于Desikan-Killiany图谱将所有受试者的纤维簇质心按连接的大脑区域分割,质心会根据其连接的脑区分为不同的子组,每个脑区的质心会随机分成10个子组,以减小计算复杂度.接着通过计算质心之间的欧几里得距离并构建亲和图,然后执行层次聚类,将每个质心簇分为多个层级,最终选择距离在一定范围内的质心进行聚类.然后,执行组间聚类以选择最具代表性的聚类,并对它们进行标记以识别连接的区域.最后,使用QuickBundles算法对标记的聚类进行最终的重组,形成清晰的纤维束,构成最终的图谱. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
... Guevara等人[49]首次在构建DWM脑图谱时涉及SWM脑图谱,他们使用凝聚平均链接层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分割,最终生成左半球47个SWM束的图谱,然后根据连接区域的功能手动标记这些图谱.Román等人[50]同样使用分层聚类的方法和欧几里得距离测量对整个大脑纤维束进行分割以创建图谱,与Guevara等人的工作不同的是,该研究专注于SWM,获得的SWM图谱中左半球有44个束,右半球有49个束,其中33个束分布在两个半球.Guevara等人[56]在聚类方法的基础上引入自动选取ROI算法,通过这种混合的方法以确定跨受试者的可重复、定义明确的束,最终创建了一个包含100个可重复束的SWM图谱,其中35个是两个半球共有的.Zhang等人[52]为了创建一个精细的全脑纤维束图谱,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,最终创建出一个包括58个DWM纤维束和198个短程和中程的SWM纤维束图谱,并且通过不同类型的受试者进行了验证,证明了该图谱的可重复性.Román等人[57]在先前工作的基础上,引入自动选取ROI的方法,与聚类方法相结合,最终创建的SWM图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI对,141束连接相同ROI的部分.5种SWM纤维束图谱的构建方法详细对比如表2所示. ...
... Comparison of SWM fiber tract atlas construction methods
Table 2 | 第一作者 | 图谱构成 | 构建数据 | 测试数据 | 纤维束追踪方法 | 概率性/确定性追踪 | 构建方法 |
Guevara[49]
| 36个DWM束,94个SWM束 | 12 NMR
| 20 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Román[50]
| 93个SWM束
| 74 CONNECT/Archi
| 78 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Zhang[52]
| 58 DWM束,198个SWM束
| 100名健康受试者
| 584名患有多
种健康状况的
受试者 | 双张量无迹
卡尔曼滤波
| 确定性
| 谱聚类
|
Guevara[56]
| 100个SWM束
| 79 CONNECT/Archi
| 26 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 自动ROI选择+层次聚类 |
Román[57]
| 525个SWM束
| 100 HCP
| 79 HARDI
| CSD+iFOD2
| 概率性
| 自动ROI选择+FFClust聚类+层次聚类 |
4 总结与展望本文对基于dMRI的SWM纤维束研究进行了广泛的调研,从SWM纤维束追踪技术,SWM纤维束分割方法,SWM纤维束脑图谱的构建等方面,总结了SWM纤维束成像与分析方面的研究进展.通过全面调研发现SWM纤维束成像方法的研究侧重点已经发生改变,从原来使用DWM纤维束成像方法逐渐变为针对于SWM的特点设计新颖的追踪方法.SWM纤维束分割的方法不再是手动选取ROI进行分割,而是逐渐变为自动选取ROI或者使用无监督聚类的方法进行SWM纤维束分割,近年来深度学习的方法也逐渐应用于SWM纤维束的分割.在此基础上,SWM脑图谱构建也更加细化,各ROI区域的连接被深入研究,分割出的簇越来越多. ...
Superficial white matter bundle atlas based on hierarchical fiber clustering over probabilistic tractography data
4
2022
... 基于ROI选择和聚类相结合的方法是指在提前定义好的ROI区域进行聚类.ROI选择可以提取和标记有意义的纤维束区域,并加快聚类速度,聚类可以有效过滤异常值,将两者相结合可以分割出有解剖学意义的纤维束.基于这种思想,一些混合的SWM纤维束分割方法被提出,Guevara等人[56]通过基于ROI选择和聚类的算法创建了全脑的SWM纤维束图谱,首先根据Desikan-Killiany图谱对每个受试者的T1加权MRI图像进行皮层分割,接着利用皮层分割结果,识别连接不同皮层ROI的纤维,最后通过受试者内聚类和受试者间聚类创建出最终的SWM纤维束图谱.Román等人[57]同样用这种方法进行SWM纤维束图谱的构建,与上述工作不同的是该研究首先进行纤维束聚类,再根据ROI分组.具体做法是首先在每个受试者内部,使用快速纤维聚类(fast fiber clustering,FFClust)算法对纤维进行聚类,以形成紧凑的纤维簇,每组纤维代表一个区域的纤维簇,并生成每个簇的质心.其次,基于Desikan-Killiany图谱将所有受试者的纤维簇质心按连接的大脑区域分割,质心会根据其连接的脑区分为不同的子组,每个脑区的质心会随机分成10个子组,以减小计算复杂度.接着通过计算质心之间的欧几里得距离并构建亲和图,然后执行层次聚类,将每个质心簇分为多个层级,最终选择距离在一定范围内的质心进行聚类.然后,执行组间聚类以选择最具代表性的聚类,并对它们进行标记以识别连接的区域.最后,使用QuickBundles算法对标记的聚类进行最终的重组,形成清晰的纤维束,构成最终的图谱. ...
... SWM fiber tract segmentation method studies
Table 1 | 第一作者 | 研究区域 | 分割方法 | 主要连接和发现 | 定量评价 |
| 分割数量 | 准确率% |
| Catani[7] | 枕叶、颞叶 | ROI/手动选择 | 枕颞外侧区相邻回的下纵束 | 1 | / |
| Wu[14] | 颞叶、顶叶、枕叶 | ROI/手动选择 | 上纵束后段连接颞中回和颞下回的后部与角回和缘上回;垂直枕束连接下顶叶、颞叶和枕叶;新的颞顶叶连接,将颞下回、颞中回、枕颞外侧回以及枕叶下部与顶叶上部互连 | 3 | / |
| Wakana[33] | 全脑 | ROI/手动选择 | 上纵束的一部分;枕叶束 | 2 | / |
| Catani[34] | 额叶、中央沟、中央前沟、岛沟、额缘沟 | ROI/手动选择 | PrCG-PoCG,PrCG-MFG,SFG-IFG,SFG-MFG,FOP,FMT,FSL,FIL,Ins-Or/Tr/Op/PrCG/SuCG | 13 | / |
| Rojkova[35] | 额叶 | ROI/手动选择 | 连接中央前回和中央后回的U型纤维;额叶斜束;连接额叶和岛叶的五个U型纤维;额叶上纵束和下纵束;额哐束和额边缘束 | 30 | / |
| Burks[36] | 顶下小叶 | ROI/手动选择 | 连接缘上回和角回的U型纤维;连接颞上沟边缘正下方和颞叶的U型纤维;连接侧裂末端和额叶的U型纤维 | 3 | / |
| Catani[37] | 顶叶 | ROI/手动选择 | SMG-SPL,AG-SPL,PoCG-AG,PoCG-SMG,PoCG-SPL,AG-SMG,SMG-SMG,aPrCu-pPrCu,SPL的前后连接和内外侧连接 | 9 | / |
| Shinohara[38] | 全脑 | ROI/手动选择 | 脑回内和脑回间U型纤维从各个方向汇聚到白质脊的交界处,构成了“金字塔形交叉” | / | / |
| Oishi[39] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,psaf | 4 | / |
| Zhang[40] | 全脑 | ROI/自动选择 | SFG-IFG,MFG-PrCG,PrCG-PoCG,SPG-SMG,SPG-PoCG,SPG-AG,SPG-PrCu,SPG-SOG,SPG-MOG,CG-SFG,CG-PrCu,SFG-MFG,SFG-PrCG,MFG-IFG,IFG-PrCG,PoCG-SMG,AG-MOG,AG-SMG,Cu-LG,Cu-SOG,Cu-MOG,FuG-IOG,FuG-MOG,SOG-MOG,IOG-MOG,STG-MTG,STG-SMG,ITG-MTG,LFOG-MFOG | 29 | / |
| Pardo[41] | 全脑 | ROI/自动选择 | 研究其SWM束的变异性 | 80 | / |
| Ouyang[42] | 全脑 | ROI/自动选择 | 没有特定的束,短关联纤维根据它们连接的两个相邻回进行分组 | / | / |
| Movahedian[43] | 初级和次级视觉皮层区域 | ROI/自动选择 | 初级和次级视觉皮层区域的短关联纤维束连接 | / | / |
| Vergani[44] | 辅助运动区 | ROI/半自动选择 | SMA-PrCG,SMA-CG | 2 | / |
| Magro[45] | 中央前回和中央后回 | ROI/半自动选择 | 中央前回和中央后回9条纤维束 | 9 | / |
| Guevara[47] | 全脑 | 流线标记/几何距离 | 在中央沟和颞上沟发现了不同人群的纤维组织的变异性 | / | / |
| Vindas[48] | 全脑 | 流线标记/几何距离 | 所提出的方法在两个数据集中都发现了更多的SWM纤维束 | / | / |
| Zhang[13] | 中央沟、中央前沟、中央后沟、颞上沟、额下沟和顶内沟 | 流线标记/聚类 | 三种数据类型共有:SFG-MFG,MFG-IFG,PrCG-PoCG,SPG-IFG,PoCG-SPG;DSI:MFG-IPL,SFG-IPL,MFG-SMG,IFG-MTG,PoCG-IPL,SPG-SMG,SMG-MTG,MTG-ITG,IPL-MOG,SFG-SPG,MFG-MTG,IFG-SPG,PrCG-SPG,SPG-IPL,SPG-SOG,SPG-MTG,STG-MTG;
HARDI:SPG-SMG,MFG-MTG,SFG-IFG,SOG-MOG,MFG-PrCG,PoCG-SMG,SPG-MTG,STG-MTG,SPG-IPL,SMG-PrCG,IPL-SMG,IPL-MTG;
DTI:SFG-IFG,MFG-PrCG,IFG-PrCG,SMG-MTG,PrCG-SPG,SFG-PrCG,SFG-PoCG,SFG-SPG,PrCG-MTG,SPG-SOG,IPL-MTG,IFG-STG,PoCG-IPL,PrCG-IPL,PoCG-IPL,IPL-MOG,SMG-MOG,STG-SMG | / | / |
| Guevara[49] | 全脑 | 流线标记/聚类 | 左半球与右半球一致:SFG-IFG(ant,mid,post),SFG-MFG(ant,mid,post),MFG-IFG,MFG(mid,mid2,post,post2),IFG-Ins,IFG(post,inf),LFOG(inf,sup),MFOG,MFOG-CG,SFG-CG(mid),MFG-PrCG(sup,mid),PrCG-PoCG(sup,inf),PrCG-Ins,PrCG-SMG,PaCG-PrCu,PoCG-SMG,SMG,SPG,AG(sup,inf),STG-AG,MTG-AG,STG(post),MTG-Ins,STG-Ins,ITG-MOG,Cu,Cu-Li,LG,FuG(ant,mid,post),PrCu-CG,PrCu-SFG,CG(ant,mid,post) | 94 | / |
| Román[50] | 全脑 | 流线标记/聚类 | 两半球共有:SPL_SPL_0i,PrCG_SFG_0i,PoCG_PrCG_0-3i,Op_SFG_0i,CMFG_PrCG_0-1i,MTG_MTG_0-1i,PrCG_SMG_0-1i,CMFG_CMFG_0i,FuG_ITG_0i,IPL_SPL_0i,MTG_STG_0i,LorFG_LorFG_0i,CMFG_Op_0i,RMFG_SFG_0-1i,Tr_SFG_0i,SMG_SMG_0-2i,RMFG_RMFG_0-1i,PoCG_SMG_0i,FuG_FuG_0i,STG_STG_0i,Tr_RMFG_0i,LOG_LOG_0-1i,
左半球:ITG_ITG_0-1l,SFG_SFG_0l,FuG_FuG_1l,PrCG_PrCG_0l,STG_STG_1l,Cu_LG_0l,PrCu_PrCu_0l,MTG_MTG_1l,LOG_LOG_2l,PrCG_Ins_0l
右半球:Tr_Tr_0r,Tr_Ins_0r,MTG_MTG_0r,SFG_SFG_1-2r,RMFG_SFG_0r,RMFG_RMFG_0-1r,PoCG_PoCG_1r,PoCG_PrCG_1r,SPL_SPL_0r,PrCu_PrCu_0r,IPL_LOG_0r,IPL_IPL_0r,LoFG_LoFG_1r,Tr_SFG_1r | 左:44;
右:49 | / |
| Zhang[52] | 全脑 | 流线标记/聚类 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | / |
| Pron[53] | 中央沟 | 流线标记/聚类 | 左半球五条U型纤维连接中央前回和中央后回 | 左:5 | / |
| Pron[54] | 中央沟 | 流线标记/聚类 | 左右半球各有五条U型纤维连接中央前回和中央后回 | 左:5;右:5 | / |
| Zhang[15] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 98.42 |
| Xue[55] | 全脑 | 流线标记/深度
学习 | 198短纤维簇连接:颞叶、顶叶-颞叶、顶叶-枕叶、顶叶、枕颞叶、枕叶、额叶-顶叶和额叶区域 | 198 | 96.79 |
| Guevara[56] | 全脑 | ROI选择和聚
类相结合 | 两半球共有:CACG-PrCu_0,CMFG-PrCG_0-1,CMFG-RMFG_0,CMFG-SFG_0,IC-PrCu_0,IPL-ITG_0,IPL-MTG_0,IPL-SMG_0,IPL-SPL_0,LOFG-RMFG_0-1,LOFG-STG_0,MOFG-STG_0,MTG-SMG_0,MTG-STG_0,Op-Ins_0,Op-PrCG_0,Op-SFG_0,Or-Ins_0,PoCiG-PrCu_1,PoCiG-RACG_0,PoCG-PrCG_0-2,PoCG-SMG_0,PrCG-Ins_0,PrCG-SMG_0,RMFG-SFG_0-1,SMG-Ins_0,SPL-SMG_0,STG-TTG_0,Tr-Ins_0,Tr-SFG_0
左半球:CMFG-Op_0,CMFG-PoCG_0,Fu-LOG_0,IPL-LOG_1,IPL-SPL_1,ITG-MTG_0,LOFG-Or_0,PoCG-Ins_0,PoCiG-PrCu_0,PoCiG-SFG_0,PoCG-PrCG_3,PoCG-SMG_1,PrCG-SFG_0,RACG-SFG_1,STG-Ins_0
右半球:CACG-PoCiG_0,CMFG-SFG_1,Cu-LG_0,Fu-LOG_1,IPL-LOG_0,ITG-MTG_1-2,LOFG-MOFG_0,LOG-SPL_0,Op-Tr_0,PoCiG-PrCu_2,PoCG-SPL_0,PrCG-SPL_0,RACG-SFG_0 | 100 | / |
| Román[57] | 全脑 | ROI选择和聚
类相结合 | 图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI部分,141束连接相同ROI部分 | 525 | / |
3 SWM纤维束图谱的构建人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
... Guevara等人[49]首次在构建DWM脑图谱时涉及SWM脑图谱,他们使用凝聚平均链接层次聚类和纤维欧几里得距离测量来对整个大脑中的短关联纤维进行分割,最终生成左半球47个SWM束的图谱,然后根据连接区域的功能手动标记这些图谱.Román等人[50]同样使用分层聚类的方法和欧几里得距离测量对整个大脑纤维束进行分割以创建图谱,与Guevara等人的工作不同的是,该研究专注于SWM,获得的SWM图谱中左半球有44个束,右半球有49个束,其中33个束分布在两个半球.Guevara等人[56]在聚类方法的基础上引入自动选取ROI算法,通过这种混合的方法以确定跨受试者的可重复、定义明确的束,最终创建了一个包含100个可重复束的SWM图谱,其中35个是两个半球共有的.Zhang等人[52]为了创建一个精细的全脑纤维束图谱,通过群组纤维束配准和谱聚类方法,将全脑纤维束分割成多个纤维聚类,最终创建出一个包括58个DWM纤维束和198个短程和中程的SWM纤维束图谱,并且通过不同类型的受试者进行了验证,证明了该图谱的可重复性.Román等人[57]在先前工作的基础上,引入自动选取ROI的方法,与聚类方法相结合,最终创建的SWM图谱由整个大脑的525束短关联纤维组成,其中384束连接不同ROI对,141束连接相同ROI的部分.5种SWM纤维束图谱的构建方法详细对比如表2所示. ...
... Comparison of SWM fiber tract atlas construction methods
Table 2 | 第一作者 | 图谱构成 | 构建数据 | 测试数据 | 纤维束追踪方法 | 概率性/确定性追踪 | 构建方法 |
Guevara[49]
| 36个DWM束,94个SWM束 | 12 NMR
| 20 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Román[50]
| 93个SWM束
| 74 CONNECT/Archi
| 78 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 层次聚类
|
Zhang[52]
| 58 DWM束,198个SWM束
| 100名健康受试者
| 584名患有多
种健康状况的
受试者 | 双张量无迹
卡尔曼滤波
| 确定性
| 谱聚类
|
Guevara[56]
| 100个SWM束
| 79 CONNECT/Archi
| 26 HARDI
| Q-ball+正则化
粒子轨迹 | 确定性
| 自动ROI选择+层次聚类 |
Román[57]
| 525个SWM束
| 100 HCP
| 79 HARDI
| CSD+iFOD2
| 概率性
| 自动ROI选择+FFClust聚类+层次聚类 |
4 总结与展望本文对基于dMRI的SWM纤维束研究进行了广泛的调研,从SWM纤维束追踪技术,SWM纤维束分割方法,SWM纤维束脑图谱的构建等方面,总结了SWM纤维束成像与分析方面的研究进展.通过全面调研发现SWM纤维束成像方法的研究侧重点已经发生改变,从原来使用DWM纤维束成像方法逐渐变为针对于SWM的特点设计新颖的追踪方法.SWM纤维束分割的方法不再是手动选取ROI进行分割,而是逐渐变为自动选取ROI或者使用无监督聚类的方法进行SWM纤维束分割,近年来深度学习的方法也逐渐应用于SWM纤维束的分割.在此基础上,SWM脑图谱构建也更加细化,各ROI区域的连接被深入研究,分割出的簇越来越多. ...
Construction of human brain templates with diffusion tensor imaging data: A review
1
2018
... 人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...
扩散张量成像的人脑模板构建
1
2018
... 人脑图谱为研究者提供了详细的大脑结构和功能分布图,使得研究者能够更准确地定位和分析不同脑区的结构和功能特性[58].SWM脑图谱是一种新颖的脑图谱,它对于研究SWM的微观结构和疾病诊断有着重要意义,其构建流程如图3所示. ...