引言
手性是自然界中普遍存在的现象,从分子氨基酸和纳米级DNA螺旋到肉眼可见的人手都具有手性. 手性分子的结构镜像对称但是无法重合,它们具有相同的组成元素和官能团,表现出许多相同的物理性质,但是在生物和药理活性上可能具有不同甚至相反的性能,因此快速鉴别手性分子对于加速药物发现、手性催化剂的筛选具有重要意义[1,2].目前手性分析的方法有很多,包括圆二色谱[3,4]、荧光法[5,6]、高效液相色谱法[7-
在过去的十多年中,用于分析手性分子的探针、介质和实验方法迅速发展[22]. 新型手性衍生化试剂(CDAs)、手性溶剂化试剂(CSAs),以及利用1H、13C、19F、31P和77Se NMR谱立体识别和确定有机化合物绝对构型的检测方法逐步成熟,NMR可以借助耦合常数、化学位移、核Overhauser效应(Nuclear Overhauser Effect,NOE)、扩散系数、弛豫时间等参数来研究分子的手性特点[22
近几年,手性硫脲因其在各种不对称反应中的催化作用备受关注[30
1 实验部分
1.1 仪器与试剂
RS-扁桃酸(RG,99%)、R-扁桃酸(RG,99%)、S-扁桃酸(RG,99%)、α-溴苯乙酸(RG,99%)、RS-α-甲氧基苯基乙酸(RG,98%)、4-三氟甲基扁桃酸(RG,98%)、2-氯丙酸(RG,98%)、2-羟基-3-甲基丁酸(RG,98%)、2-苯基丙酸(RG,98%)、2-羟基-3-甲基丁酸(RG,98%)、苯甘氨醇(RG,98%)、氧化苯乙烯(RG,98%)、L-苯丙氨酸甲酯盐酸盐(H-Phe-OMe•HCl,RG,99%)、1,8-二氮杂双环[5.4.0]十一碳-7-烯(DBU,RG,98%)、4-二甲氨基吡啶(DMAP,RG,98%)购自阿达玛斯(Adamas)公司. L-丙氨酸甲酯盐酸盐(H-Ala-OMe•HCl,RG,99%)、L-缬氨酸甲酯盐酸盐(H-Val-OMe•HCl,RG,99%)购自吉尔吉化(上海)有限公司,4-三氟甲基苯异氰酸酯(RG,99%)购自TCI公司,二氯甲烷、乙酸乙酯、环己烷购自天津市北联精细化学品开发有限公司,甲醇钾购自北京伊诺凯科技有限公司,三乙胺购自天津市富宇精细化工有限公司.
NMR实验在AVANCE NEO 400 MHz NMR谱仪(瑞士Bruker公司)上进行.
1.2 实验过程
1.2.1 手性脲的合成
L-Phe-U、L-Val-U和L-ALa-U的合成过程参见文献[41].
1.2.2 手性识别的1H NMR实验
在1H NMR手性识别测定实验中,将手性羧酸底物溶解于CDCl3(以TMS为内标,δH 0 ppm)中,配制成溶液,加入CSAs形成一定比例的主客体系,随后在液体NMR波谱仪测定不同体系的1H NMR谱. 1H NMR的工作频率为400.12 MHz,实验温度为25℃,弛豫时间为2 s,累加次数16,采样点数为2 048. 实验步骤如下:
(1)先称取RS-扁桃酸(rac-MA)1.4 mg溶于500 μL CDCl3,置于NMR样品管中,采集1H NMR谱;随后按照摩尔比rac-MA : L-Phe-U = 1: 1加入L-Phe-U,再次采集1H NMR谱;在此基础上,按照摩尔比rac-MA : L-Phe-U : DMAP =1 : 1 : 1,加入DMAP,采集1H NMR谱. 3次1H NMR谱做对比,观察CSAs对R型和S型扁桃酸的识别能力.
(2)rac-MA和DMAP量不变,改变L-Phe-U的浓度(0.25,0.5,0.75,2.0,3.0,4.0 equiv.)重复步骤(1)中的实验.
(3)将手性底物分子换成α-溴苯乙酸、4-三氟甲基扁桃酸、2-苯基丙酸、2-氯丙酸、苯甘氨醇等,重复步骤(1)中的实验,观察手性化学位移试剂对不同手性底物的识别能力.
(4)在rac-MA : L-Phe-U : DMAP = 1: 3: 1的基础上,将L-Phe-U换成L-Ala-U以及L-Val-U,相同条件下采集1H NMR谱,比较不同CSAs的对映体识别能力.
(5)称取2 mg rac-MA溶于500 μL CDCl3中,设置DOSY实验参数;采集完之后,加入L-Phe-U使得rac-MA : L-Phe-U的摩尔比为1 : 3,再次采集DOSY实验;实验结束后,加入DMAP使得rac-MA : L-Phe-U : DMAP的摩尔比为1: 3: 1,采集DOSY实验. 将三次DOSY实验数据进行拟合,获得rac-MA的CαH信号在不同体系里的扩散系数并进行分析.
1.2.3 NMR扩散排序谱(DOSY)实验
DOSY实验使用Bruker公司的标准实验脉冲序列:ledbpgp2s,谱宽是3 601.08 Hz,激发中心为1 600.48 Hz,1H通道射频脉冲脉宽为10 μs,功率为20.07 W,弛豫延迟时间为(D1)为2 s,累加次数为64,脉冲序列中的扩散时间为10 ms,梯度脉冲宽度分别为2 320 μs、3 000 μs、3 450 μs,每个DOSY实验梯度脉冲的强度变化范围为2%~95%,采用线性模式在该范围内选取24个变化值,采样数据点为2 048.
所有实验数据处理软件为Bruker公司提供的Topspin 4.20,通过其中的dynamics模块程序对数据进行处理,选择扁桃酸CαH为目标,对该峰进行积分,拟合.
2 结果与讨论
2.1 含氨基酸单元手性脲对rac-MA的手性识别
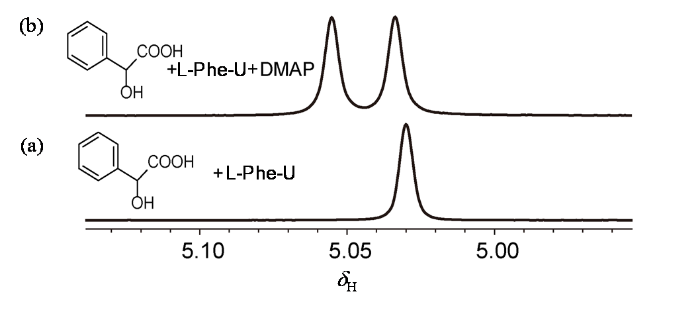
本文中我们选择了手性识别研究中常用的不同结构的手性羧酸作为底物,以此来探究含不同氨基酸结构单元手性脲(图1)的对映体识别能力. 首先,选用L-Phe-U作为CSAs,以rac-MA为手性客体底物,展开实验. 在NMR实验过程中,以rac-MA的甲基质子(CαH)信号变化为依据,通过观察R型与S型化学位移差(ΔΔδ = |ΔδS-ΔδR|),来判断L-Phe-U的对映体识别能力. 我们将10 μmol/L rac-MA溶于500 μL氘代氯仿(CDCl3)中,按照摩尔浓度比1 : 1加入L-Phe-U,在1H NMR谱图上并没有观察到rac-MA CαH的化学位移变化[图2(a)]. 宋玲课题组在2013年发现DMAP与手性羧酸通过NH…O键形成carboxylate-DMAPH+ 离子对,加强了CSAs和手性客体底物之间的氢键相互作用,可以帮助区分羧酸的对映体[42]. 我们按照一定比例加入DMAP(L-Phe-U : DMAP : rac-MA = 1 : 1 : 1),发现rac-MA的CαH出现两个信号,ΔΔδ = 8.8 Hz[图2(b)],说明DMAP在手性识别过程中发挥积极作用,与L-Phe-U和rac-MA之间有相互作用.
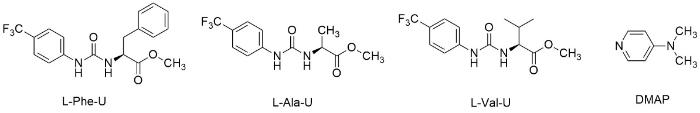
图1
图1
手性化学位移试剂(L-Phe-U、L-Ala-U和L-Val-U)以及4-二甲氨基吡啶(DMAP)结构
Fig. 1
Structure of chiral chemical shift reagent (L-Phe-U, L-Ala-U, L-Val-U) and DMAP
图2
图2
rac-MA(10 μmol/L,CDCl3)苯基CαH在不同条件下的1H NMR图. (a)加入10 μmol/L L-Phe-U;(b)加入10 μmol/L L-Phe-U和10 μmol/L DMAP
Fig. 2
Partial 1H NMR spectra showing the benzylic C-H protons of rac-MA (10 μmol/L, CDCl3) in the presence of (a) L-Phe-U (10 μmol/L) and (b) L-Phe-U (10 μmol/L) and DMAP (10 μmol/L)
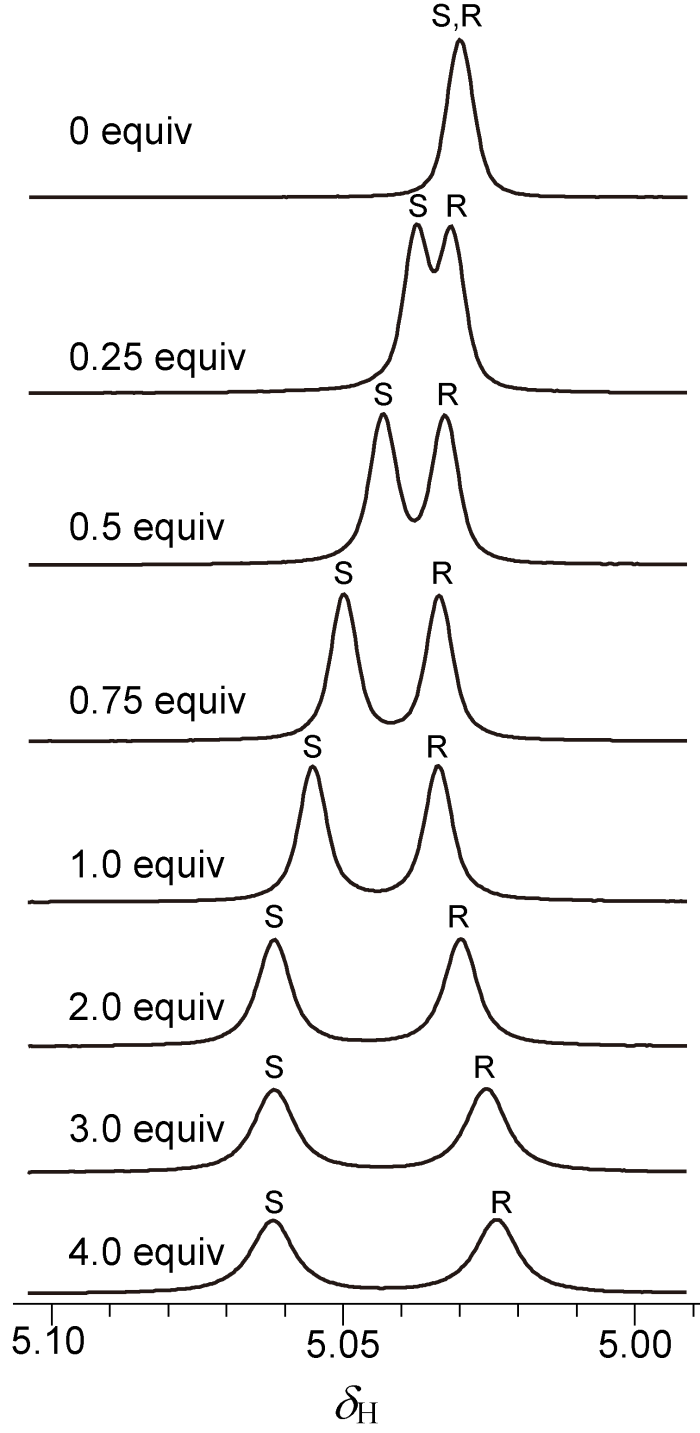
随后我们进行了CSAs浓度滴定实验,随着L-Phe-U量的不断增加,rac-MA CαH质子对映体信号化学位移差逐渐增大. 当L-Phe-U : DMAP : rac-MA = 3 : 1 : 1时,ΔΔδ = 15.2 Hz,继续滴加时,化学位移差仍略有增加(ΔΔδ = 16.0 Hz)(图3),但是变化幅度减小,这说明当L-Phe-U的浓度高于30 μmol/L时,其对rac-MA的手性识别能力不再随浓度的增大而显著增强.
图3
图3
在DMAP作用下rac-MA(10 μmol/L,CDCl3)CαH随着L-Phe-U浓度变化(0~4.0当量)的1H NMR谱图
Fig. 3
1H NMR spectra of the benzylic C-H protons of rac-MA (10 μmol/L) with 0~4.0 equivalents of L-Phe-U in the presence of DMAP (in CDCl3)
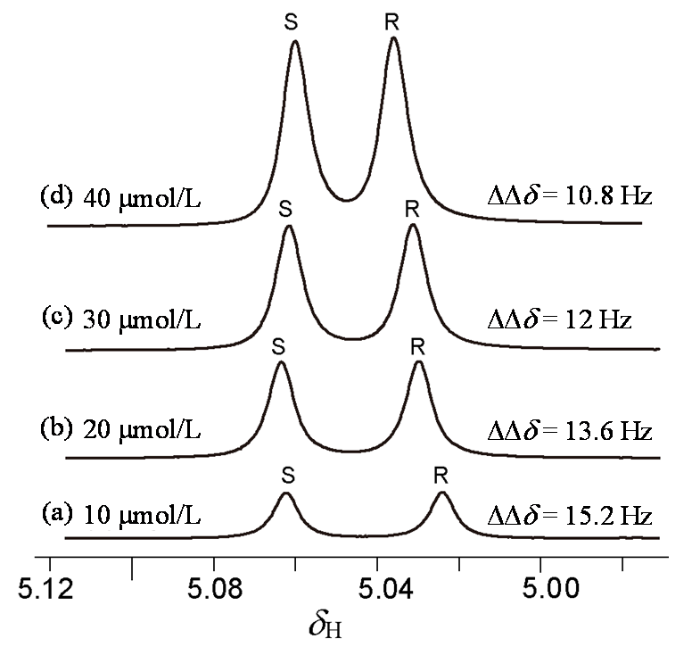
在同样的条件下(L-Phe-U : DMAP : rac-MA = 3 : 1 : 1),逐渐增加rac-MA的含量(图4),发现随着rac-MA含量的增加,ΔΔδ逐渐减小(10 μmol/L:ΔΔδ = 15.2 Hz;40 μmol/L:ΔΔδ = 10.8 Hz).这些结果显示,在L-Phe-U与MA以及DMAP三者之间存在着动力学平衡,当三者之间的浓度(比例)发生变化时,这种平衡受到干扰,从而影响对映体的识别.
图4
图4
在L-Phe-U和DMAP(3 : 1)作用下,不同浓度的rac-MA CαH的1H NMR谱图(CDCl3)
Fig. 4
1H NMR spectra of the benzylic C-H protons of rac-MA mixtures with varying concentrations in the presence of 3.0 equivalents of L-Phe-U and 1.0 equivalent of DMAP in CDCl3
2.2 不同CSAs与手性羧酸的相互作用
挑选不同的手性羧酸客体底物(溴苯乙酸、2-苯基丙酸、4-三氟甲基扁桃酸、2-甲氧基苯基乙酸、2-氯丙酸、苯甘氨醇和氧化苯乙烯),检验L-Phe-U作为CSAs的对映体识别能力. 按照L-Phe-U : 客体底物 : DMAP = 3 : 1 : 1的条件进行实验,发现L-Phe-U对不同手性客体底物的识别能力不同. 如表1所示,L-Phe-U对rac-MA有很好的识别作用(ΔΔδ = 15.2 Hz)(表1,序号1),当扁桃酸苯基对位氢被三氟甲基取代时,ΔΔδ可以达到9.6 Hz(表1,序号2),其CαH化学位移差较扁桃酸CαH有所降低,但是仍能很好的区分R, S构型. 对于2-苯基丙酸而言ΔΔδ只能达到2.4 Hz(表1,序号3),但是当2-苯基丙酸的甲基被吸电子基团Br取代时,CαH R型和S型的化学位移差增大(ΔΔδ = 11.2 Hz,表1,序号4).当扁桃酸羟基氢被甲基取代时,CαH对映体的化学位移差仅有0.64 Hz,L-Phe-U结构上的甲基氢与其信号重叠,影响判断(表1,序号5).Dogan课题组指出,手性底物的苯环结构以及DMAP吡啶环结构之间的π-π堆积相互作用影响对映体之间的手性识别[26]. 为了进一步验证这一推论,我们选择了没有苯基结构的2-氯丙酸,发现L-Phe-U也有一定的识别能力,ΔΔδ可达2.4 Hz(表1,序号6),但是当客体底物为苯甘氨醇和氧化苯乙烯时,L-Phe-U没有任何的手性识别能力.
表1 不同的手性脲存在条件下,外消旋羧酸CαH的化学位移差值(ΔΔδ)(CDCl3)
Table 1
| 序号 | 手性羧酸 | 手性化学位移试剂 | 谱图 | ΔΔδ/Hz |
|---|---|---|---|---|
| 1 | 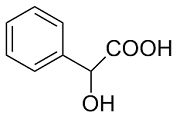 | L-Phe-U |  | 15.2 |
| 2 | 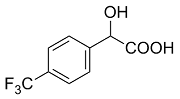 | L-Phe-U | 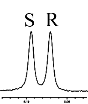 | 9.6 |
| 3 | 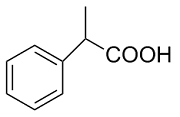 | L-Phe-U | 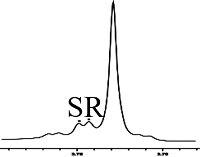 | 2.4 |
| 4 | 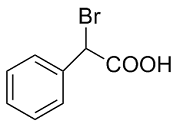 | L-Phe-U | 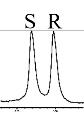 | 11.2 |
| 5 | 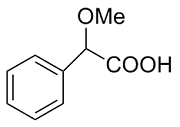 | L-Phe-U |  | 0.64 |
| 6 | 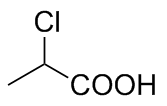 | L-Phe-U | 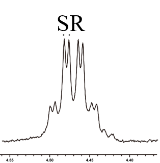 | 2.4 |
| 7 | 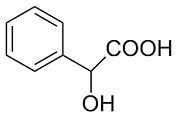 | L-ALa-U | 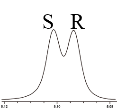 | 3.2 |
| 8 | 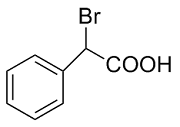 | L-ALa-U |  | 4.0 |
| 9 | 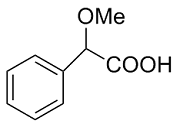 | L-ALa-U |  | 3.6 |
为了对比不同的CSAs对手性客体底物的识别能力,我们选择了另外两种含氨基酸结构的手性脲(L-ALa-U和L-Val-U)进行实验,结果发现这两种CSAs的对映体识别能力比L-Phe-U弱.在DMAP作用条件下,当CSAs为L-ALa-U时,rac-MA的化学位移差ΔΔδ仅有3.2 Hz(表1,序号7),当扁桃酸上的羟基被极性基团Br取代时,ΔΔδ有所增加,R型和S型扁桃酸被区分开(ΔΔδ = 4.0 Hz,表1,序号8);2-甲氧基苯基乙酸的对映体也能够被识别,其ΔΔδ = 3.6 Hz(表1,序号9). 同样条件下用L-Val-U作为CSAs进行实验,发现其对rac-MA无手性识别能力. 通过比较实验结果我们推测CSAs的苯基结构以及客体底物手性碳上连接的基团会影响两者之间的手性识别,此类脲对手性羧酸对映体的选择效果比较明显. 手性识别是一个相对比较复杂的过程,手性化学位移试剂、底物以及加入体系中其他辅助识别的物质结构都会对这一过程造成影响. L-Phe-U手性识别效果优于其它两个取代的氨基酸硫脲的原因是由于苯环的环电流屏蔽效果较好,因此选择具有更大环电流屏蔽作用的氨基酸如色氨酸合成手性化学位移试剂,可能有助于手性羧酸的识别.目前带有色氨酸及其衍生物结构的化合物已被用于扁桃酸的手性识别[43],非天然氨基酸取代基的双硫脲手性识别体系已有报道[44],我们后续研究将在此基础上继续探究带有不同氨基酸结构的手性化学位移试剂对手性识别的影响.
2.3 手性识别测定以及动力学研究
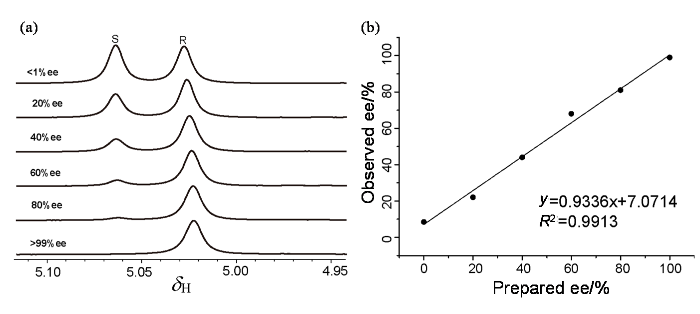
图5
图5
(a)在L-Phe-U和DMAP作用下,不同ee值的R-扁桃酸、S-扁桃酸的1H NMR谱;(b)实验设计ee值与实验检测ee值的线性相关图
Fig. 5
(a) Selected region of the 1H NMR spectra of mandelic acid of various enantiomeric impurities in the presence of L-Phe-U and DMAP; (b) Correlation between prepared and observed ee values obtained by 400 MHz 1H NMR titrations of enantiomerically enriched mixtures of mandelic acid using L-Phe-U and DMAP in CDCl3
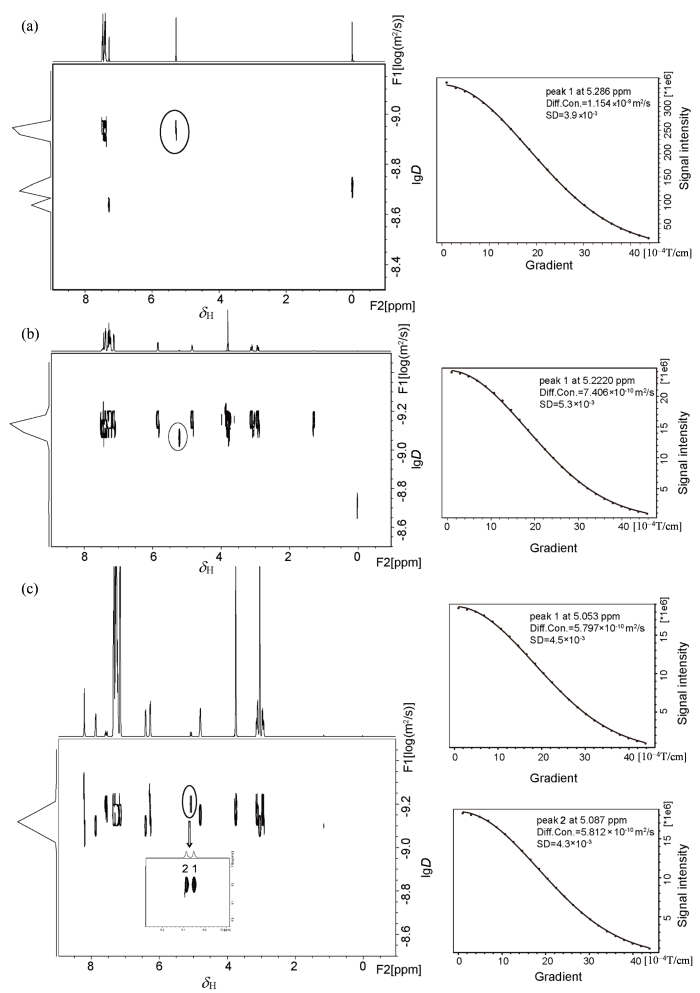
除此之外,我们分别测试了rac-MA体系、rac-MA/L-Phe-U二元体系以及rac-MA/L-Phe-U/DMAP三元体系的NMR扩散排序谱(DOSY),比较了CαH的扩散系数. 单一的rac-MA的CαH(12 μmol/L)扩散系数(diffusion coefficient)为1.154×10-9 m2/s[图6(a)],当加入3 equiv. L-Phe-U时,其扩散系数为 7.406×10-10 m2/s,CαH的扩散系数明显减小,但是R型和S型并没有被区分开[图6(b)],继续加入1 equiv. DMAP,此时CαH手性被识别. 分别对R型和S型信号进行拟合,发现R型的CαH扩散系数为 5.797×10-10 m2/s,S型的CαH扩散系数为5.812×10-10 m2/s,两者的扩散运动存在差异[图6(c)]. 通过DOSY实验我们发现CSAs会减慢手性分子的运动,这种状态更有利于对映体被识别.
图6
图6
在相同扩散时间和不同扩散梯度强度(2%~95%)的情况下,(a) rac-MA体系;(b) rac-MA/L-Phe-U二元体系;(c) rac-MA/L-Phe-U/DMAP三元体系CαH扩散系数拟合曲线(Diff. Con.指扩散系数,SD指拟合的标准偏差)
Fig. 6
Simulated diffusion decay curves of CαH (black circle) in (a) rac-MA, (b) rac-MA and L-Phe-U, (c) rac-MA, L-Phe-U and DMAP by varying the gradient strength from 2% to 95% with the same diffusion time and different diffusion gradient strength (Diff. Con. is for diffusion coefficient, and SD is for standard deviation)
3 结论
我们利用NMR技术研究了三种含氨基酸结构单元CSAs的对映体识别能力,其中L-Phe-U对所选的手性羧酸底物识别性能比较好. 羧酸底物手性中心上所连接的基团以及CSAs结构都会影响对映体R型和S型信号的区分. CSAs会影响对映体的运动,通过DOSY实验发现,扁桃酸R型和S型对映体的扩散系数变小,尽管两者存在的差异性比较小,但其手性依然能够被区分. 含氨基酸结构单元的手性脲合成过程简单、稳定性强,能够准确地用于对映体的定量分析,我们将继续研究这类物质,期待能够用于更多手性底物对映体的识别.
参考文献
Zn(II) promoted dramatic enhancement in the enantioselective fluorescent recognition of functional chiral amines by a chiral aldehyde
[J].
Enantioseparation on poly(phenyl isocyanide)s with macromolecular helicity memory as chiral stationary phases for HPLC
[J].
Circular dichroism of multi-component assemblies for chiral amine recognition and rapid determination
[J].
Chiral recognition a stereo dynamic vanadium probe using the electronic circular dichroism effect in differential Raman scattering
[J].
Enantioselective fluorescent sensors: A tale of BINOL
[J].
Enantioselective recognition of carboxylic acids by novel fluorescent triazine-based thiazoles
[J].
DOI:10.1002/chir.22792
PMID:29210117
[本文引用: 1]

Hydrogen bonding and π-π interactions take special part in the enantioselectivity task. In this regard, because of having both hydrogen acceptor and hydrogen donor groups, melamine derivatives become more of an issue for enantioselectivity. In the light of such information, triazine-based chiral, fluorescence active novel thiazole derivatives L1 and L2 were designed and synthesized from (S)-(-)-2-amino-1-butanol and (1S,2R)-(+)-2-amino-1,2-diphenylethanol. The structural establishment of these compounds was made by spectroscopic methods such as FTIR, H, and C NMR. While the solution of these compounds in DMSO did not show any fluorescence emission, it was observed that the emission increased 44-fold for L1 and 55-fold for L2 in 95% water, similar to the aggregation-induced emission (AIE) characterized compounds. In this regard, enantioselective capabilities of these compounds against carboxylic acids were tested, and in experiments carried out at a ratio of 40/60 DMSO/H O, it was determined that R-2ClMA increased the fluorescence emission of L1 chiral receptor by 2.59 times compared to S-isomer.© 2017 Wiley Periodicals, Inc.
Recent developments in preparative-scale supercritical fluid- and liquid chromatography for chiral separations
[J].
Chiral metal-organic frameworks-based materials for chromatographic enantioseparation
[J].
DOI:10.1016/j.cclet.2024.109787

The homochiral compounds play an important role in human health and pharmaceutical industry. Currently, the chromatographic enantioseparation has become one of the most effective and practical approach to obtain pure enantiomers. Herein, the exploration of advanced materials, using as chromatographic chiral stationary phases for racemic separation, has attracted great attention. Thanks to their high enantioselectivity and controllable synthesis, the emerging chiral metal-organic frameworks (CMOFs) have been widely studied as the stationary phase in chromatographic technology. In this review, we will summarize the principles of synthetic strategies and mechanism of chiral microenvironment. In particular, the recent progress and research hotspot of CMOFs regarding as the chiral stationary phases in gas chromatography (GC), high-performance liquid chromatography (HPLC), and capillary electrochromatography (CEC), are elucidated systematically according to the published work. Last but not the least, we also highlight the challenges and perspectives of rational design of CMOFs, as well as their corresponding racemic separation. We envision that the review will provide a further understanding of CMOFs and facilitate the development of chromatographic enantioselective applications.
Green HPLC enantioseparation of chemopreventive chiral isothiocyanates homologs on an immobilized chiral stationary phase based on amylose tris-[(S)-α-methylbenzylcarbamate]
[J].
Chromatographic separation of R/S-enantiomers of amphetamine and methamphetamine: Pathways of methamphetamine synthesis and detection in blood samples by qualitative enantioselective LC-MS/MS analysis
[J].
DOI:S0379-0738(18)30533-4
PMID:30199817
[本文引用: 1]

Methamphetamine can be synthesized either enantiopure or in its racemic form. We separated (R)- and (S)-enantiomers of methamphetamine and amphetamine by a fast LC-MS/MS-method using a Lux 3μm AMP 150×3.0mm analytical column after simple protein precipitation with methanol. Sufficient resolution could be achieved. Method validation for qualitative detection showed limits of quantification <5ng/mL while only little (maximum 14.5%) ion suppression could be shown. Stability in the processed sample could be achieved using isotopically labelled internal standards. Plasma samples of police cases from the german regions of Franconia and Northrhine revealed that in the majority of 106 tested samples (>99%) only (S)-methamphetamine was detected which leads to the conclusion that, in Germany, predominantly enantiopure (S)-methamphetamine is consumed which is synthesized via (1R,2S)-ephedrine or (1S,2S)-pseudoephedrine. However, racemic methamphetamine seems also to be on the market.Copyright © 2018 Elsevier B.V. All rights reserved.
Enantiomeric analysis of chiral drugs using mass spectrometric methods: A comprehensive review
[J].
Recent trends in chiral separation-a collective paradigm of selected chiral impurities
[J].
Recent progress for chiral stationary phases based on chiral porous materials in high-performance liquid chromatography and gas chromatography separation
[J].
1H NMR chiral analysis of charged molecules via ion pairing with aluminum complexes
[J].
Recent advances in multinuclear NMR spectroscopy for chiral recognition of organic compounds
[J].
Direct chiral discrimination with NMR
[J].
Determining the absolute configuration of small molecules by diffusion NMR experiments
[J].
Enantiodifferentiation of chiral diols and diphenols via recognition-enabled chromatographic 19F NMR
[J].
Mechanisms underlying enantiomeric discrimination of its structural analogues with a diphenylethylenediamine derivative revealed by proton NMR spectroscopy
[J].
核磁共振氢谱中二苯基乙二胺衍生物手性识别其结构类似物
[J].
DOI:10.11938/cjmr20182703
[本文引用: 1]

本文通过手性二苯基乙二胺与异氰酸酯的衍生化反应,合成了一种C<sub>2</sub>对称的手性主体1.该主体可以手性识别其结构类似物:α-苯乙胺(客体2)、α-对甲氧基苯乙胺(客体9)以及它们的衍生物(客体3~8和10~13).高分辨核磁共振氢谱(<sup>1</sup>H NMR)显示了对映体识别中主客体间的氢键作用.结果表明,除含2个NO<sub>2</sub>的客体7和12外,主体1可以较易识别含有两个伯胺的脲和酰胺衍生物.研究还发现,主体1对脲衍生物2、9比对酰胺衍生物有更强的氢键作用,此外主体1对(R)和(S)-脲衍生物中的CHCH<sub>3</sub>基团也有更高的辨识能力.
Using NMR spectroscopic methods to determine enantiomeric purity and assign absolute stereochemistry
[J].DOI:10.1016/j.pnmrs.2010.07.003 PMID:21600355 [本文引用: 1]
A bisthiourea-based 1H NMR chiral sensor for chiral discrimination of a variety of chiral compounds
[J].
Chirality sensing of tertiary alcohols by a novel strong hydrogen-bonding donor-selenourea
[J].
New chiral oxo-bridged calix[2]arene[2]triazine for the enantiomeric recognition of alpha-racemic carboxylic acids
[J].
Differentiation of enantiomeric anions by NMR spectroscopy with chiral bisurea receptors
[J].
DOI:10.1039/c7ob02318a
PMID:29136083

Chiral anionic species are ubiquitous and play important roles in biological systems. Despite the recent advancements in synthetic anion receptors bearing urea functionalities, urea-based chiral solvating agents (CSAs) that can separate the NMR signals of racemic anions remain limited. Herein, three dibenzofuran-based C-symmetric chiral bisureas were synthesized from the reaction of (R,R)-4,6-bis(1-aminopropyl)dibenzo[b,d]furan with phenyl isocyanate, phenyl thioisocyanate, or tosyl isocyanate. The chiral anion recognition properties of these bisureas were examined by H NMR spectroscopy using dl-tetrabutylammonium mandelate (TBAM) as a model substrate. A clear baseline separation of the enantiomeric signals of the benzylic proton of TBAM was achieved upon mixing with 0.5 equivalents of bis(phenylurea). In contrast to previous urea-based chiral anion receptors that differentiate the enantiomers of chiral anions by forming 1 : 1 host-guest complexes, a high chiral recognition ability of chiral bis(phenylurea) was achieved owing to the generation of an equilibrium between free guests, 1 : 1 host-guest complexes, and 1 : 2 host-guest complexes. Chiral bis(phenylurea) was also successfully employed in the separation of the enantiomeric H NMR signals of various racemic anions.
Enantiodiscrimination of carboxylic acids using single enantiomer thioureas as chiral solvating agents
[J].
Chiral NMR solvating additives for differentiation of enantiomers
[J].
Thiourea derivative of 2-[(1R)-1-Aminoethyl]phenol: a flexible pocket-like chiral solvating agent (CSA) for the enantiodifferentiation of amino acid derivatives by NMR spectroscopy
[J].
Asymmetric organocatalytic reactions by bifunctional amine-thioureas
[J].
Bifunctional primary amine-thioureas in asymmetric organocatalysis
[J].
DOI:10.1039/c3ob41403e
PMID:24057617

Research disclosed since the demonstration of the first examples of primary amine-thiourea organocatalysis in 2006 has shown that primary amine-based thioureas can successfully catalyze a diverse variety of highly enantioselective transformations providing a wide range of versatile organic compounds. Recent remarkable progress with these chiral catalysts is summarized in this review.
Facile synthesis of chiral cyclic ureas through hydrogenation of 2-hydroxypyrimidine/pyrimidin-2(1h)-one tautomers
[J].
Highly enantioselective synthesis of acyclic N,N'-acetals by chiral urea derived from quinine catalyzed the addition of aryl amines to isatin-derived ketimines
[J].
A dimeric thiourea CSA for the enantiodiscrimination of amino acid derivatives by NMR spectroscopy
[J].
DOI:10.1021/acs.joc.1c00340
PMID:34019407
[本文引用: 1]

The reaction of benzoyl isothiocyanate with (1,2)-1,2-bis(2-hydroxyphenyl)ethylenediamine afforded a new thiourea chiral solvating agent (CSA) with a very high ability to differentiate H and C NMR signals of simple amino acid derivatives, even at low concentrations. The enantiodiscrimination efficiency was higher with respect to that of the parent monomer, a thiourea derivative of 2-((1)-1-aminoethyl)phenol, thus putting into light the relevance of the cooperativity between the two molecular portions of the dimer in a cleft conformation stabilized by interchain hydrogen bond interactions. An achiral base additive (DABCO or DMAP) played an active role in the chiral discrimination processes, mediating the interaction between the CSA and the enantiomeric mixtures. The chiral discrimination mechanism was investigated by NMR spectroscopy through the determination of complexation stoichiometries, association constants, and the stereochemistry of the diastereomeric solvates.
Enantioselective sensing of carboxylic acids with a bis (urea) oligo (phenylene) ethynylene foldamer
[J].
Bis-thiourea chiral sensor for the NMR enantiodiscrimination of N-acetyl and N-trifluoroacetyl amino acid derivatives
[J].
Thiourea organocatalysts as emerging chiral pollutants: en route to porphyrin-based (chir) optical sensing
[J].
A Thiourea derivative of 2-[(1)-1-aminoethyl]phenol as a chiral sensor for the determination of the absolute configuration of N-3,5-dinitrobenzoyl derivatives of amino acids
[J].
Chiral recognition of ibuprofen enatiomers by a chiral thiourea in the presence of DMAP using NMR
[J].
手性硫脲在DMAP条件下对布洛芬类药物的NMR手性识别
[J].
DOI:10.11938/cjmr20140410
[本文引用: 1]

通过<sup>1</sup>H NMR 研究了手性硫脲对布洛芬类药物的手性识别.在4-二甲氨基吡啶(DMAP)的参与下,硫脲对布洛芬类化合物显示出良好的识别效果,并且布洛芬对映异构体的α-H NMR 信号与硫脲和DMAP 无相互干扰.为了检测硫脲/DMAP 对手性布洛芬对映体纯度的分析能力,测定了若干ee.(对换体过量百分数)值的布洛芬溶液,与理论值吻.
Study on synthesis and chiral recognition of chiral hosts containing amino acid unit
[J].
含氨基酸单元的手性主体的合成及手性识别研究
[J].以L-丙氨酸和2,7-二萘酚为原料通过简单的方法合成了二种带有荧光基团萘的手性阴离子主体(1和2), 用红外光谱、质谱、元素分析、核磁共振氢谱及碳谱表征了它们的结构. 用荧光光谱及核磁共振氢谱研究了主体与二苯甲酰酒石酸阴离子的相互作用, 结果表明1, 2与D-或L-二苯甲酰酒石酸阴离子均形成1∶1的配合物, 主体1展现出良好的对二苯甲酰酒石酸阴离子对映选择性识别能力.
Stereoselective ring-opening polymerization of rac-lactide using chiral urea/strong organobase binary catalyst system
[J].
手性脲/有机碱二元体系协同催化外消旋丙交酯立构选择性开环聚合
[J].
A chiral bisthiourea as a chiral solvating agent for carboxylic acids in the presence of DMAP
[J].
DOI:10.1021/jo4013546
PMID:24050150
[本文引用: 1]

A simple chiral bisthiourea has been used as a highly effective and practical chemical solvating agent (CSA) for diverse α-carboxylic acids in the presence of DMAP. Excellent enantiodiscrimination based on well-resolved α-H NMR signals of the enantiomers of carboxylic acids can be obtained without interference from the chiral bisthiourea and DMAP. To check the practicality of the chiral bisthiourea/DMAP for enantiomeric determination, the ee values of mandelic acid (MA) samples over a wide ee range were determined by integration of the α-H signal of MA in (1)H NMR. A discrimination mechanism is proposed, that the formation of two diasteromeric ternary complexes between the chiral bisthiourea and two in situ formed enantiomeric carboxylate-DMAPH(+) ion pairs discriminates the enantiomers of carboxylic acids. Computational modeling studies show that the chemical shift value of α-H of (S)-MA is greater than that of (R)-MA in ternary complexes, which is consistent with experimental observation. 1D and 2D NOESY spectra demonstrate the intermolecular noncovalent interactions between the protons on the aromatic rings of chiral bisthiourea and α-H of the enantiomers of racemic α-methoxy phenylacetic acids in the complexes.
Amino acid-based molecular and membranous chiral tools for enantiomeric recognition
[J].
Enantioselective recognition of sodium carboxylates by an 1,8-diaminoanthracene based ion pair receptor containing amino acid units
[J].