
Chinese Journal of Magnetic Resonance ›› 2021, Vol. 38 ›› Issue (2): 164-172.doi: 10.11938/cjmr20202841
• Articles • Previous Articles Next Articles
Jin-bo YU1,2,Cai ZHANG1,2,Ze-ting ZHANG1,Guo-hua XU1,*( ),Cong-gang LI1
),Cong-gang LI1
Received:2020-07-07
Published:2021-06-05
Online:2020-09-01
Contact:
Guo-hua XU
E-mail:guohua_xu@wipm.ac.cn
CLC Number:
Jin-bo YU,Cai ZHANG,Ze-ting ZHANG,Guo-hua XU,Cong-gang LI. Interactions Between α-synuclein and Intact Mitochondria Studied by NMR[J]. Chinese Journal of Magnetic Resonance, 2021, 38(2): 164-172.
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
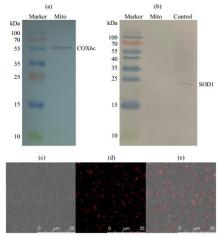
Fig.1
The analysis of isolated mitochondrial purity and viability from the liver of rat. (a) The COX6c component and (b) SOD1 component were detected by western blotting (SOD1 standard sample as positive control in figure (b)), showing the mitochondrial purity. (c)~(e) Mitochondrial viability characterized by fluorescence confocal microscopy: (c) Bright field; (d) Isolated mitochondria were stained with Mito Tracker® Red CMXRos whose accumulation is dependent upon the mitochondrial membrane potential (MMP); (e) Overlay of figure (c) and figure (d)
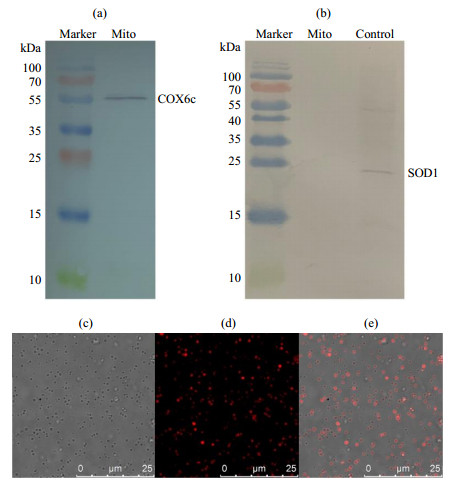
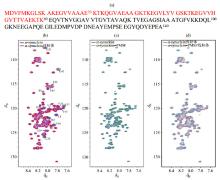
Fig.2
1H-15N SOFAST-HMQC spectra of α-synuclein under different conditions. (a) The amino acid sequence of α-synuclein, the N-terminus region (1~60) are colored in red; (b) Spectral overlay of α-synuclein in absence (blue) and presence (red) of intact mitochondria, the partial peaks of which signal intensity attenuated in the presence of intact mitochondria are marked; (c) Spectral overlay of α-synuclein with (green) and without (blue) PMSF(1 mmol/L) in diluted solution; (d) Spectral overlay of α-synuclein with (cyan) and without (red) PMSF (1 mmol/L) in the presence of intact mitochondria
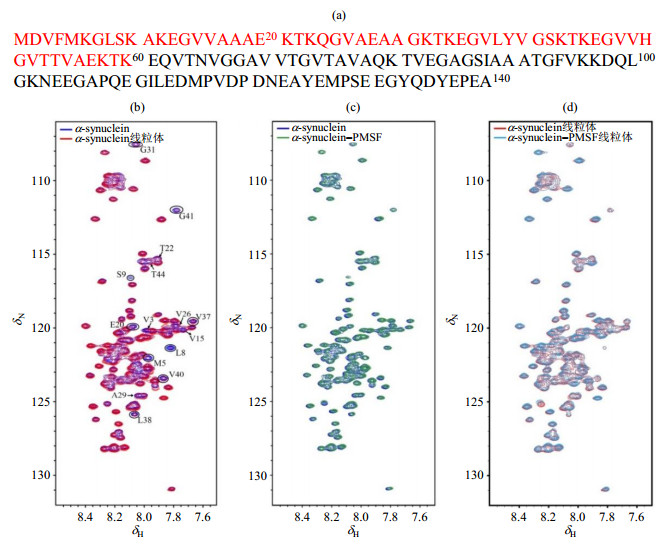
Fig.3
The analysis of residue resolved NMR signal intensity and chemical shift change of α-synuclein in the presence of mitochondria (the values of NMR signal intensity ratio and chemical shift changes are shown as blank for the residues whose signal intensity can not be accurately obtained due to the overlap of spectral peaks). (a) NMR signal intensity ratio (Iα-synuclein-mito/Iα-synuclein) of α-synuclein in the presence and absence of mitochondria; (b) Chemical shift changes of α-synuclein in presence of mitochondria compared to α-synuclein; (c) NMR signal intensity ratio (Iα-synuclein-PMSF-mito/Iα-synuclein) of α-synuclein with PMSF in the presence of mitochondria and α-synuclein; (d) Chemical shift changes of α-synuclein with PMSF in the presence of mitochondria compared to α-synuclein
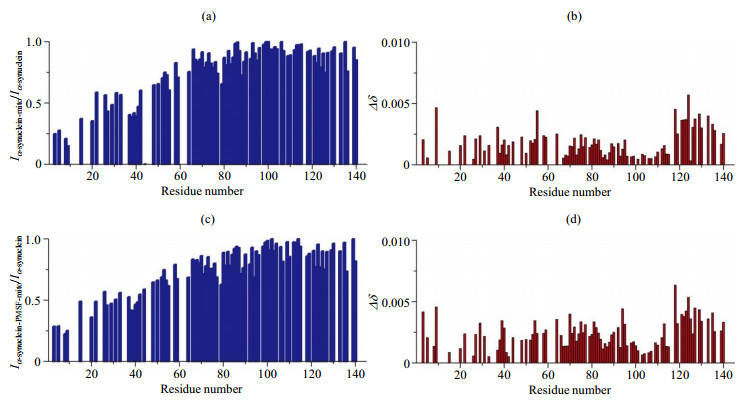
Fig.4
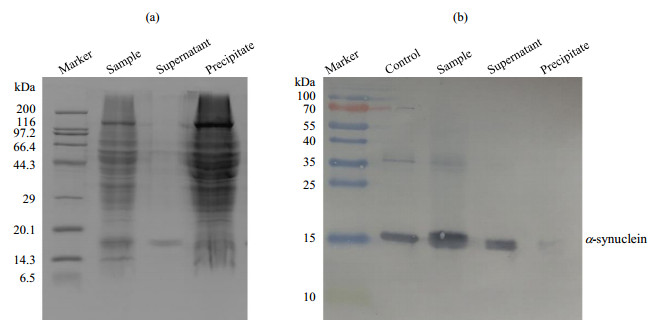
(a) SDS-PAGE and (b) Western-blotting analysis of α-synuclein with PMSF in the presence of intact mitochondria (a) SDS-PAGE analysis showing integrity of mitochondria; (b) Western-blotting analysis showing that α-synuclein is undegraded; Control: α-synuclein standard sample, Sample: α-synuclein with PMSF in the presence of intact mitochondria, Supernatant: the supernatant of the sample (α-synuclein with PMSF in the presence of intact mitochondria) that was centrifuged, Precipitate: the precipitate of the sample that was centrifuged
| 1 |
POLYMEROPOULOS M H , LAVEDAN C , LEROY E , et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease[J]. Science, 1997, 276 (5321): 2045- 2047.
doi: 10.1126/science.276.5321.2045 |
| 2 |
SPILLANTINI M G , SCHMIDT M L , LEE V M Y , et al. Alpha-synuclein in Lewy bodies[J]. Nature, 1997, 388 (6645): 839- 840.
doi: 10.1038/42166 |
| 3 |
MASLIAH E , ROCKENSTEIN E , VEINBERGS I , et al. Dopaminergic loss and inclusion body formation in alpha-synuclein mice: Implications for neurodegenerative disorders[J]. Science, 2000, 287 (5456): 1265- 1269.
doi: 10.1126/science.287.5456.1265 |
| 4 |
FINK A L . The aggregation and fibrillation of alpha-synuclein[J]. Accounts Chem Res, 2006, 39 (9): 628- 634.
doi: 10.1021/ar050073t |
| 5 |
ZAMMIT V A , RAMSAY R R , BONOMINI M , et al. Carnitine, mitochondrial function and therapy[J]. Adv Drug Deliver Rev, 2009, 61 (14): 1353- 1362.
doi: 10.1016/j.addr.2009.04.024 |
| 6 |
PALADE G E . The fine structure of mitochondria[J]. Anat Record, 1952, 114 (3): 427- 451.
doi: 10.1002/ar.1091140304 |
| 7 |
FREY T G , MANNELLA C A . The internal structure of mitochondria[J]. Trends Biochem Sci, 2000, 25 (7): 319- 324.
doi: 10.1016/S0968-0004(00)01609-1 |
| 8 |
LIN M T , CANTUTI-CASTELVETRI I , ZHENG K , et al. Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease[J]. Ann Neurol, 2012, 71 (6): 850- 854.
doi: 10.1002/ana.23568 |
| 9 |
SHAVALI S , BROWN-BORG H M , EBADI M , et al. Mitochondrial localization of alpha-synuclein protein in alpha-synuclein overexpressing cells[J]. Neurosci Lett, 2008, 439 (2): 125- 128.
doi: 10.1016/j.neulet.2008.05.005 |
| 10 |
LI L , NADANACIVA S , BERGER Z , et al. Human A53T alpha-synuclein causes reversible deficits in mitochondrial function and dynamics in primary mouse cortical neurons[J]. PloS ONE, 2013, 8 (12): e85815.
doi: 10.1371/journal.pone.0085815 |
| 11 |
PERFEITO R , LAZAR D F , OUTEIRO T F , et al. Linking alpha-synuclein phosphorylation to reactive oxygen species formation and mitochondrial dysfunction in SH-SY5Y cells[J]. Mol Cell Neurosci, 2014, 62, 51- 59.
doi: 10.1016/j.mcn.2014.08.002 |
| 12 |
GANJAM G K , BOLTE K , MATSCHKE L A , et al. Mitochondrial damage by alpha-synuclein causes cell death in human dopaminergic neurons[J]. Cell Death Dis, 2019, 10 (11): 865- 879.
doi: 10.1038/s41419-019-2091-2 |
| 13 |
SCHAPIRA A H V . Mitochondria in the aetiology and pathogenesis of Parkinson's disease[J]. Lancet Neurol, 2008, 7 (1): 97- 109.
doi: 10.1016/S1474-4422(07)70327-7 |
| 14 |
MIZUNO Y , IKEBE S , HATTORI N , et al. Role of mitochondria in the etiology and pathogenesis of Parkinson's disease[J]. Biochim Biophys Acta, 1995, 1271 (1): 265- 274.
doi: 10.1016/0925-4439(95)00038-6 |
| 15 |
ZIGONEANU I G , YANG Y J , KROIS A S , et al. Interaction of alpha-synuclein with vesicles that mimic mitochondrial membranes[J]. BBA-Biomembranes, 2012, 1818 (3): 512- 519.
doi: 10.1016/j.bbamem.2011.11.024 |
| 16 |
ROBOTTA M , GERDING H R , VOGEL A , et al. Alpha-synuclein binds to the inner membrane of mitochondria in an alpha-helical conformation[J]. Chembiochem, 2014, 15 (17): 2499- 2502.
doi: 10.1002/cbic.201402281 |
| 17 |
ROBOTTA M , HINTZE C , SCHILDKNECHT S , et al. Locally resolved membrane binding affinity of the N-terminus of alpha-synuclein[J]. Biochemistry, 2012, 51 (19): 3960- 3962.
doi: 10.1021/bi300357a |
| 18 | GAO D L , SUN P , WANG Q W , et al. Interactions between albumin and fatty acids studied by NMR spectroscopy[J]. Chinese J Magn Reson, 2018, 35 (3): 338- 344. |
| 高东莉, 孙鹏, 王倩文, 等. 运用NMR研究白蛋白与脂肪酸的相互作用[J]. 波谱学杂志, 2018, 35 (3): 338- 344. | |
| 19 | NING C F , MA M J , GUO C H , et al. Interactions between 5-fluorouracil and PAMAM dendrimers studies by NMR spectroscopy[J]. Chinese J Magn Reson, 2019, 36 (4): 555- 562. |
| 宁彩芳, 马敏珺, 郭朝晖, 等. PAMAM树状大分子与5-氟尿嘧啶相互作用的NMR研究[J]. 波谱学杂志, 2019, 36 (4): 555- 562. | |
| 20 |
THEILLET F X , BINOLFI A , BEKEI B , et al. Structural disorder of monomeric alpha-synuclein persists in mammalian cells[J]. Nature, 2016, 530 (7588): 45- 50.
doi: 10.1038/nature16531 |
| 21 |
ZHENG W W , ZHANG Z T , YE Y S , et al. Phosphorylation dependent alpha-synuclein degradation monitored by in-cell NMR[J]. Chem Commun, 2019, 55 (75): 11215- 11218.
doi: 10.1039/C9CC05662A |
| 22 |
YE Y S , LIU X L , XU G H , et al. Direct observation of Ca2+-induced calmodulin conformational transitions in intact xenopus laevis oocytes by 19F NMR spectroscopy[J]. Angew Chem Int Edit, 2015, 54 (18): 5328- 5330.
doi: 10.1002/anie.201500261 |
| 23 |
XU G H , YE Y S , LIU X L , et al. Strategies for protein NMR in escherichia coli[J]. Biochemistry, 2014, 53 (12): 1971- 1981.
doi: 10.1021/bi500079u |
| 24 |
YE Y S , LIU X L , CHEN Y H , et al. Labeling strategy and signal broadening mechanism of protein NMR spectroscopy in xenopus laevis oocytes[J]. Chemistry, 2015, 21 (24): 8686- 8690.
doi: 10.1002/chem.201500279 |
| 25 |
YE Y S , LIU X L , ZHANG Z T , et al. 19F NMR spectroscopy as a probe of cytoplasmic viscosity and weak protein interactions in living cells[J]. Chem-Eur J, 2013, 19 (38): 12705- 12710.
doi: 10.1002/chem.201301657 |
| 26 |
HOYER W , ANTONY T , CHERNY D , et al. Dependence of alpha-synuclein aggregate morphology on solution conditions[J]. J Mol Biol, 2002, 322 (2): 383- 393.
doi: 10.1016/S0022-2836(02)00775-1 |
| 27 | DAI C Y , LIU M L , LI C G . Salt content-dependent conformational changes of alpha-synuclein studied by 19F NMR[J]. Chinese J Magn Reson, 2015, 32 (1): 33- 44. |
| 戴晨晔, 刘买利, 李从刚. 低盐和高盐环境下α-synuclein构象的19F NMR研究[J]. 波谱学杂志, 2015, 32 (1): 33- 40. | |
| 28 |
COLLOMBET J M , WHEELER V C , VOGEL F , et al. Introduction of plasmid DNA into isolated mitochondria by electroporation[J]. J Biol Chem, 1997, 272 (8): 5342- 5347.
doi: 10.1074/jbc.272.8.5342 |
| 29 |
PARIHAR M S , PARIHAR A , FUJITA M , et al. Mitochondrial association of alpha-synuclein causes oxidative stress[J]. Cell Mol Life Sci, 2008, 65 (7-8): 1272- 1284.
doi: 10.1007/s00018-008-7589-1 |
| 30 |
SCHMITT S , SCHULZ S , SCHROPP E M , et al. Why to compare absolute numbers of mitochondria[J]. Mitochondrion, 2014, 19, 113- 123.
doi: 10.1016/j.mito.2014.06.005 |
| 31 |
MIWA S , TREUMANN A , BELL A , et al. Carboxylesterase converts Amplex red to resorufin: Implications for mitochondrial H2O2 release assays[J]. Free Radical Biol Med, 2016, 90, 173- 183.
doi: 10.1016/j.freeradbiomed.2015.11.011 |
| 32 |
ZHANG Z T , DAI C Y , BAI J , et al. Ca2+ modulating alpha-synuclein membrane transient interactions revealed by solution NMR spectroscopy[J]. BBA-Biomembranes, 2014, 1838 (3): 853- 858.
doi: 10.1016/j.bbamem.2013.11.016 |
| 33 |
FUSCO G , DE SIMONE A , GOPINATH T , et al. Direct observation of the three regions in alpha-synuclein that determine its membrane-bound behaviour[J]. Nat Commun, 2014, 5, 3827- 3834.
doi: 10.1038/ncomms4827 |
| 34 |
ZHANG Z T , JIANG X , XU D R , et al. Calcium accelerates SNARE-mediated lipid mixing through modulating alpha-synuclein membrane interaction[J]. BBA-Biomembranes, 2018, 1860 (9): 1848- 1853.
doi: 10.1016/j.bbamem.2018.03.025 |
| [1] | KOU Xinhui, ZHANG Yubing. Study on the Enantiomeric Recognition of Chiral Ureas Containing Amino Acid Units [J]. Chinese Journal of Magnetic Resonance, 2025, 42(3): 221-230. |
| [2] | DU Qunjie. Experimental Study on Accurate Determination of Shale Porosity by Nuclear Magnetic Resonance [J]. Chinese Journal of Magnetic Resonance, 2025, 42(3): 275-284. |
| [3] | SHEN Zhiqiang, DENG Yabo, YANG Peiju, HU Xiaoxue, HUANG Xiaojuan, XU Chuanzhi, SONG Huanling. Design and Application of an in situ NMR Device for Light-Induced Reaction Systems [J]. Chinese Journal of Magnetic Resonance, 2025, 42(1): 22-33. |
| [4] | XU Xiaojie, CHEN Yan’an, LI Xufei, ZHANG Yuncai, ZHANG Yong, ZHAN Dongkai, PAN Ting. Structural Elucidation of Hybutimibe [J]. Chinese Journal of Magnetic Resonance, 2024, 41(1): 43-55. |
| [5] | WANG Feng, LIU Tingwei, XU Yajie, YU Peng, WANG Ya, PENG Bowen, YANG Xiaodong. A Miniaturised NMR RF Probe Design with External Field-locking Channel [J]. Chinese Journal of Magnetic Resonance, 2023, 40(3): 332-340. |
| [6] | WANG Yuanfang,WANG Xiaohua,SHU Chang,ZHANG Xu,LIU Maili,ZENG Danyun. The Aggregation of ATAD2 Bromodomain in Solution [J]. Chinese Journal of Magnetic Resonance, 2023, 40(2): 169-178. |
| [7] | HE Caiyan,XIAO Yuqing,LI Shenhui,XU Jun,DENG Feng. Solid-state NMR Investigation of the Host-guest Interactions in Gas Adsorption and Chemical Separation Using MOFs as Adsorbents [J]. Chinese Journal of Magnetic Resonance, 2023, 40(2): 192-206. |
| [8] | ZHAO Chang,GONG Zhou. Investigation of Dynamic Structure of Protein Encountering Complex with Paramagnetic NMR [J]. Chinese Journal of Magnetic Resonance, 2023, 40(2): 148-157. |
| [9] | ZHAN Jianhua,HU Qin,ZHU Qinjun,JIANG Bin,ZHANG Xu,LIU Maili. Track the Conformational Change of Unlabeled Yeast Cytochrome c in Cell Homogenate Using NMR [J]. Chinese Journal of Magnetic Resonance, 2023, 40(1): 22-29. |
| [10] | CI Jie,YANG Xue,XIN Jiaxiang,WEI Daxiu,YAO Yefeng. Preparation and Lifetime Studies of the Singlet State of Five Spins in Hexene Molecules Used to Guide the Preservation of the Parahydrogen-induced Nuclear Polarization State [J]. Chinese Journal of Magnetic Resonance, 2023, 40(1): 30-38. |
| [11] | Yun-shan PEI, Cai ZHANG, Xiao-li LIU, Kai CHENG, Ze-ting ZHANG, Cong-gang LI. Inhibition of α-Synuclein Aggregation by the Interaction Between Protein Disulfide Isomerase and α-Synuclein [J]. Chinese Journal of Magnetic Resonance, 2022, 39(4): 381-392. |
| [12] | Xiao-yang ZHANG, Shou-quan YAO, Jun-cheng XU, Yu JIANG. Magnetic Field Locking System Based on Fluxgate and Time Domain Digital Frequency Discrimination [J]. Chinese Journal of Magnetic Resonance, 2022, 39(4): 448-458. |
| [13] | Han HU,Wei-yu WANG,Jun XU,Feng DENG. 1, 3-Butadienen Hydrogenation on Supported Pd-Sn Bimetallic Catalysts Investigated by Parahydrogen-induced Polarization [J]. Chinese Journal of Magnetic Resonance, 2022, 39(2): 133-143. |
| [14] | Qian XU,Lang CHEN,Xiang-ying HU,Cong-gang LI,Yi-xiang LIU,Ling JIANG. The Effect of T69E-mimicked Phosphorylation on the Interaction Between Bcl-2 and Nur77 [J]. Chinese Journal of Magnetic Resonance, 2022, 39(1): 87-95. |
| [15] | Xiao-qing LIN,Shi-jia DU,Hao-lin ZHAN,Yu-qing HUANG,Zhong CHEN. Two-Dimensional Homonuclear Orthogonal-Pattern Phase-Sensitive J-Resolved NMR Spectroscopy Based on Pure Shifts [J]. Chinese Journal of Magnetic Resonance, 2021, 38(4): 448-459. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||