引言
表1 我国已管制的12种尼秦类物质的结构式
Table 1
 |
目前,国内外关于尼秦类物质的研究集中在合成路线[7]、质谱特征规律[8,9]、检验检测方法[10]、代谢性质[11]、成瘾性危害性评估[12],完整的波谱学数据和结构解析还未见报道.本实验室近期在送检样品中检测出一种新的尼秦类化合物,与近年流行的苄基尼秦有一定差异.综合利用傅里叶变换红外光谱(FTIR)、气相色谱-质谱联用(GC-MS)、超高效液相色谱-高分辨质谱联用(UPLC-HRMS)、核磁共振(NMR)多种方法对其进行结构解析,确定结构为依托尼秦的苯乙基结构类似物2-[2-(4-ethoxyphenyl)ethyl]-N,N-diethyl-5-nitro-1H-benzimidazole-1-ethanamine (CAS:805984-43-4),简称为苯乙基依托尼秦(图1),早期的临床前研究发现其活性约为吗啡的50倍[13],具备一定的滥用风险.本研究的相关内容可以为日后工作中更多尼秦类似物的结构推断提供参考.
图1
1 实验部分
1.1 仪器与试剂
所用仪器如下:GCMS-QP 2020NX气相色谱-质谱联用仪(GC-MS),日本岛津;Thermo Orbitrap Exploris 120高分辨质谱-液相色谱质谱联用仪(UHPLC-HRMS),美国赛默飞;Bruker AVANCE NEO 600核磁共振仪,德国Bruker;PerkinElmer Spectrum 3傅里叶变换红外光谱仪(FT-IR),美国珀金埃尔默;Integrion离子色谱仪,美国赛默飞;Mettler-Toledo XSR205DU电子天平,瑞士梅特勒-托利多.
甲酸为质谱纯试剂(德国Merck Chemicals),甲醇为色谱纯试剂(德国Merck Chemicals),乙腈为色谱纯试剂(德国Merck Chemicals),氘代甲醇-d4(CD3OD,99.8% D + 0.03% (v/v) TMS)和氘代氯仿(CDCl3,99.8% D + 0.03% (v/v) TMS)采购自美国剑桥同位素(CIL),离子色谱(IC)测试所用Cl标准溶液购自赛默飞(美国Dionex 氯化物标准品1 000 mg/L),超纯水由Millipore IQ7000超纯水仪(美国密理博)制取.
1.2 样品来源及制备
黄色粉末样品为地方公安部门查获的可疑毒品样品.
取适量黄色粉末,使用衰减全反射附件(Attenuated Total Reflectance,ATR)直接用粉末进行IR分析.
取约10 mg样品置于离心管中,加入10 mL甲醇,超声溶解,涡旋混匀,0.45 μm滤膜过滤,得到溶液待分析用.取100 μL上述溶液用900 μL甲醇稀释后进行GC-MS分析,取20 μL上述溶液用490 μL甲醇和490 μL水溶液稀释后进行UPLC-HRMS分析.
取约10 mg样品,使用氘代甲醇-d4或者氘代氯仿充分溶解后进行NMR分析.
取约10 mg样品,使用10 mL纯化水溶解后进行离子色谱分析.
1.3 测定条件
1.3.1 FTIR测试条件
FTIR使用ATR测试,扫描次数为4,分辨率为4 cm-1,扫描范围为4 000~400 cm-1.
1.3.2 GC-MS测试条件
使用SH-Rxi-5Sil MS(30 m × 0.25 mm × 0.25 µm毛细柱)色谱柱.升温程序如下:初始温度140 ℃,保持3 min,以20 ℃/min速率升温至320 ℃,保持13 min,进样口温度280 ℃,传输线温度250 ℃,电子轰击(Electron Ionization,EI)离子源温度230 ℃.载气使用高纯氦气,柱流量为1 mL/min,采用分流进样方式,进样量为1 μL,分流比设为40 : 1.使用全扫描方式采集数据,质量扫描范围35~500 amu,设置溶剂延迟2 min.
1.3.3 UPLC-HRMS测试条件
UPLC测试条件如下:选用Hypersil GOLD C18(2.1 mm×100 mm,1.9 μm)色谱柱;柱温为35 ℃;采用梯度洗脱方式,其中流动相为A相为0.01%甲酸水溶液,B相为甲醇,流速为0.4 mL/min,洗脱程序设定为:0~2 min,5%B;2~9.5 min,5%B~80%B;9.5~12 min,80%B;12~12.1 min,80%B~5%B;12.1~15 min,5%B.进样量:1 μL.
HRMS测试条件如下:采用电喷雾离子化(Electrospray Ionization,ESI)正负离子模式,设置源电压为3.5 kV,辅助气压力10 psi,鞘气压力45 psi,温度320 ℃,扫描范围(m/z)为150~1 500,分辨率为120 000.二级条件设置为:隔离窗(m/z)为1,采用高能碰撞解离(HCD)模式进行离子碎裂,碰撞能量(CE)分别取10、20、40、60 eV,静电场轨道阱(Orbitrap)分辨率为60 000.
1.3.4 NMR测试条件
NMR实验均在Bruker AVANCE NEO 600MHz核磁共振波谱仪上完成.样品溶于CD3OD或CDCl3,以TMS为内标(δH 0,δC 0),1H NMR 和13C NMR的工作频率分别为600.18和150.93 MHz,实验温度为23 ℃,谱宽分别为11 904.8和35 714.3 Hz.二维谱包括1H-1H COSY、1H-1H NOESY、1H-1H TOCSY、1H-13C HSQC及1H-13C HMBC谱,均采用标准脉冲程序.1H-1H COSY的F2(1H)和F1(1H)维的谱宽均为5 882.4 Hz,采样数据点阵t2×t1 = 1 024×128,累加次数1.1H-1H NOESY的F2(1H)和F1(1H)维的谱宽均为5 882.4 Hz,采样数据点阵t2×t1 = 1 024×256,累加次数4.1H-1H TOCSY的F2(1H)和F1(1H)维的谱宽均为5 882.4 Hz,采样数据点阵t2×t1 = 1 024×256,累加次数8.1H-13C HSQC的F2(1H)和F1(13C)维的谱宽分别为5 882.4 Hz和24 902.9 Hz,采样数据点阵t2×t1= 512×256,累加次数2.1H-13C HMBC的F2(1H)和F1(13C)维的谱宽分别为5 263.2 Hz 和33 204.7 Hz,采样数据点阵t2×t1 = 2 048×128,累加次数8.
1.3.5 离子色谱测试条件
阴离子色谱具体分析条件如下:选用Dionex IonPac AS11-HC-4μm阴离子交换柱色谱柱(4 mm × 250 mm),柱温为30 ℃.淋洗液由Dionex EGC 500 KOH淋洗液发生器在线生成25 mmol/L KOH溶液,以等度洗脱模式输送,流速恒定为1.0 mL/min;检测系统配备Dionex ADRS 600(4 mm)阴离子抑制器,抑制电流为62 mA,通过电导检测器定量分析目标离子;进样量为50 μL.
2 结果与讨论
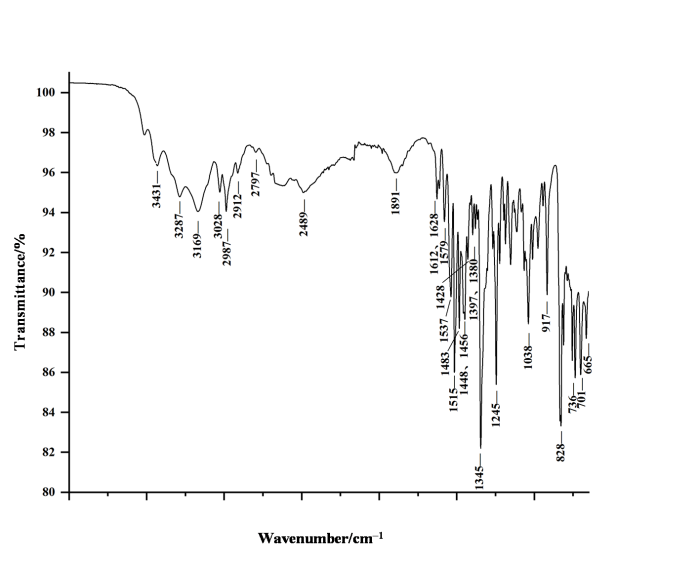
2.1 IR光谱分析
苯乙基依托尼秦IR(图2)中最强的1 515 cm−1和1 345 cm−1分别是芳香硝基N-O键不对称和对称的伸缩振动引起的[14-
图2
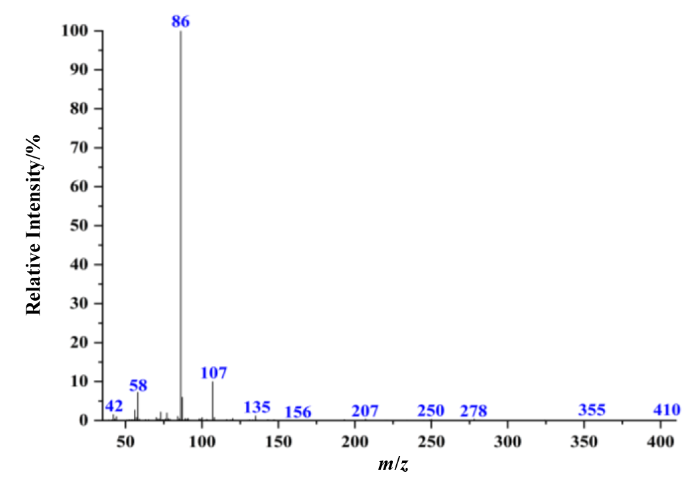
2.2 电子轰击质谱(EI-MS)谱图分析
图3
图4
图4
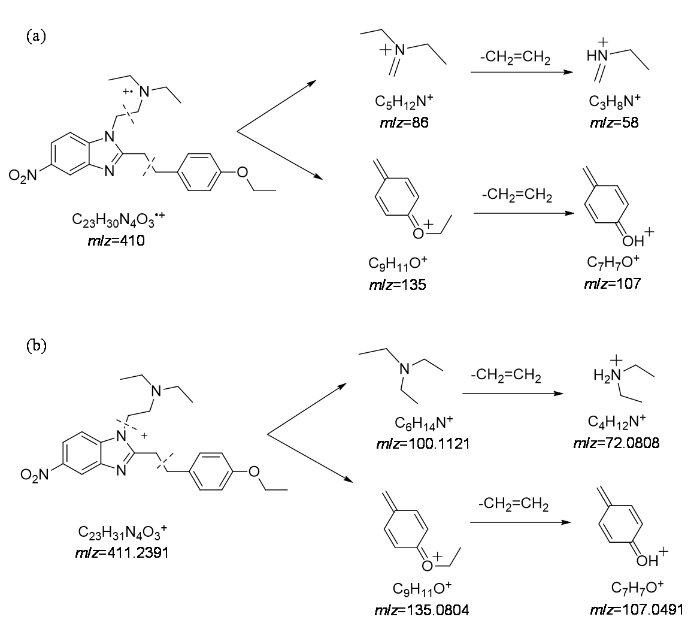
苯乙基依托尼秦可能的(a) EI碎裂规律和(b) ESI碎裂规律
Fig. 4
Possible EI fragmentation patterns (a) and ESI fragmentation patterns (b) of phenylethyl etonitazene
2.3 ESI-HRMS谱图分析
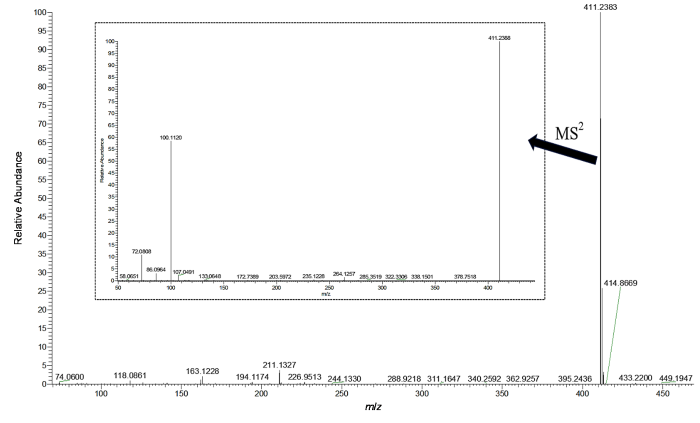
ESI-HRMS的一级谱图(图5)中检出苯乙基依托尼秦的准分子离子峰[M+H]+ m/z 411.238 3,推测其化学式为C23H31N4O3+.准分子离子峰的测量值(411.238 3)与理论值(411.239 1)偏差为1.9×10-6,符合通用允许偏差5×10-6;此外同位素峰蔟412.241 8(25.32%)和413.245 1(3.0%)符合理论预测的同位素分布412.242 5 (24.9%)、413.245 8 (3.0%),进一步验证了化学式正确.二级谱图(图5插图)中m/z 100.112 0为烷氨基侧链离子,其进一步丢失CH2=CH2生成碎片离子m/z 72.080 8[图4(b)],同时谱图中还检出了与EI质谱图中相同的苄基侧链碎片离子m/z 135.080 4、107.049 1,表明EI谱中m/z 135和107碎片离子结构指认正确.
图5
图5
苯乙基依托尼秦的ESI-HRMS谱图(MS1和MS2)
Fig. 5
ESI-HRMS spectrum (MS1 and MS2) of phenylethyl etonitazene
2.4 NMR谱图分析
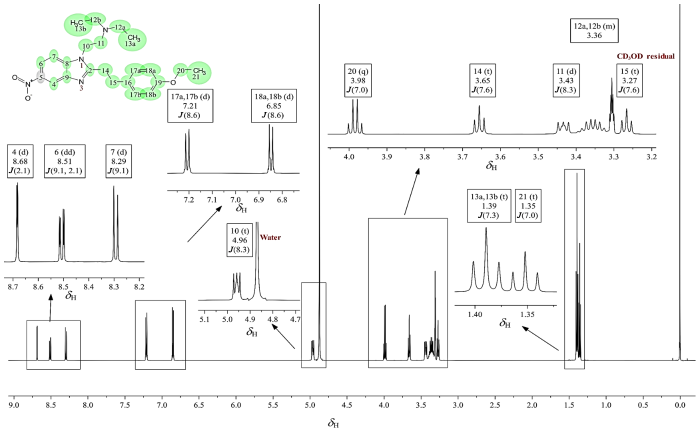
图6
图6
苯乙基依托尼秦的1H NMR谱(CD3OD)
Fig. 6
1H NMR spectrum of phenylethyl etonitazene (CD3OD)
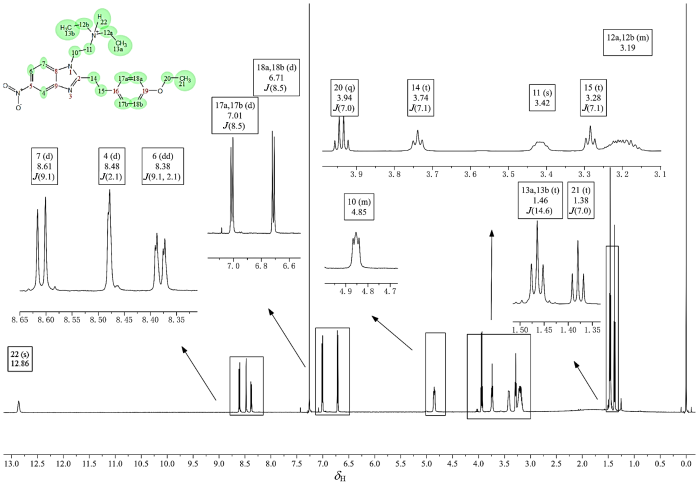
图7
图7
苯乙基依托尼秦的1H NMR谱(CDCl3)
Fig. 7
1H NMR spectrum of phenylethyl etonitazene (CDCl3)
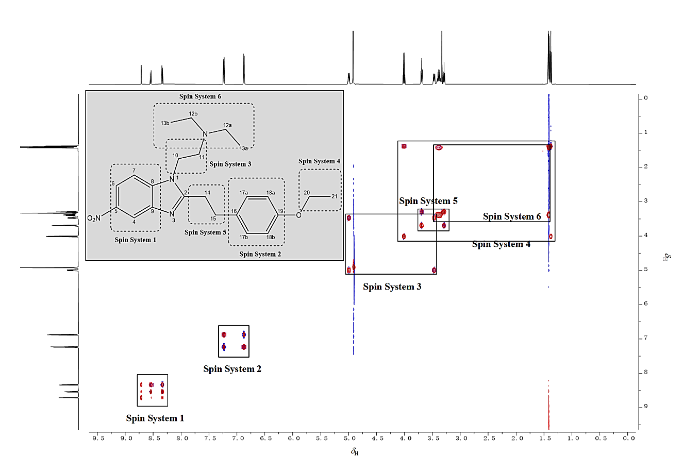
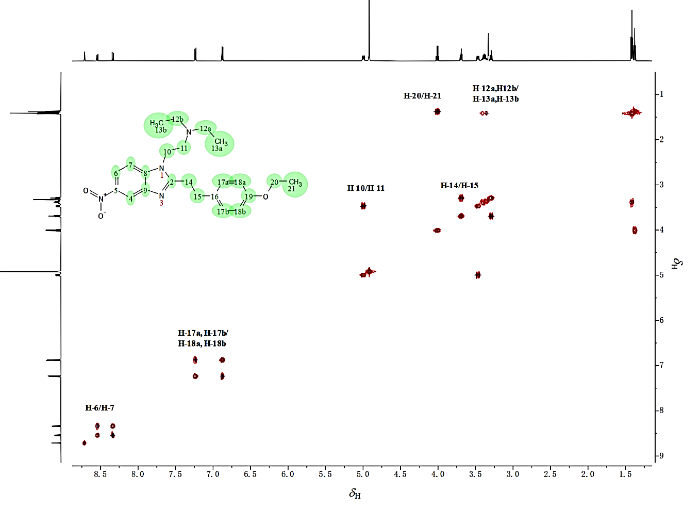
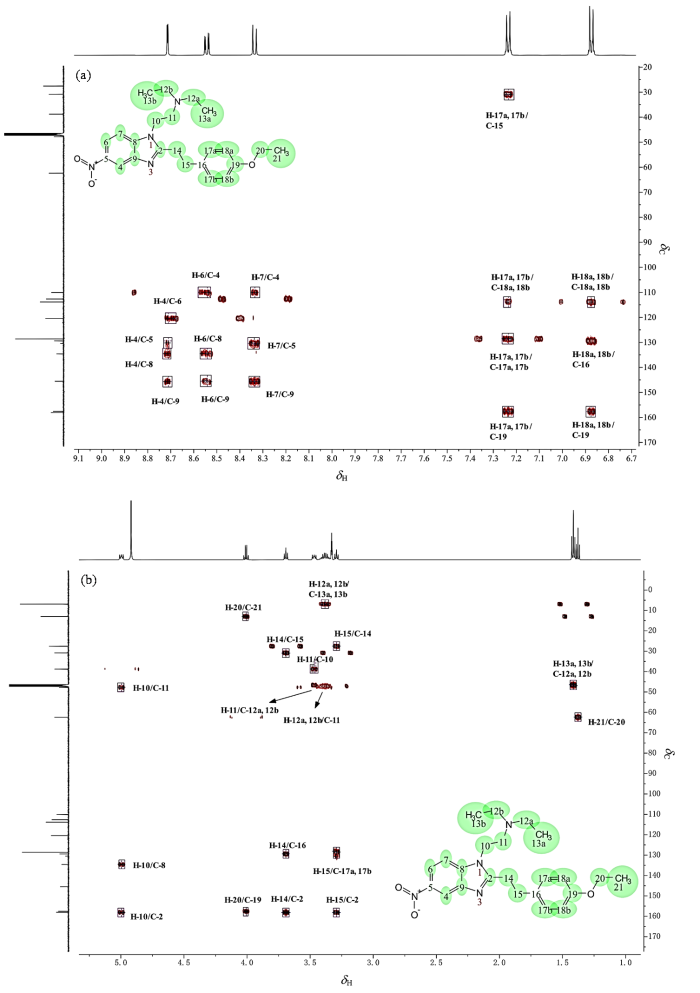
通过1H-1H TOCSY谱(图8),可将苯乙基依托尼秦氢谱中的氢原子区分为6个不同的自旋体系,分别对应不同的结构片段,根据化学位移规律、耦合分裂情况以及二维谱,按照氢谱的化学位移顺序解析如下[14
图8
图8
苯乙基依托尼秦的1H-1H TOCSY谱图和6个典型自旋体系(CD3OD)
Fig. 8
1H-1H TOCSY spectrum of phenylethyl etonitazene and six characteristic spin systems (CD3OD)
第2耦合系统是由δH 7.21 (2H, d, J = 8.6 Hz) 和δH 6.85 (2H, d, J = 8.6 Hz) 构成的苯环典型AA'BB'系统,OCH2CH3属于给电子的邻对位定位基,故间位的电子云密度低,化学位移高,按照化学位移规律将其分别归属为H-17a、H-17b和H-18a、H-18b.第3耦合系统是乙二胺片段中两个亚甲基δH 4.96 (2H, t, J = 8.3 Hz) 和δH 3.43 (2H, t, J = 8.3 Hz) 构成的一组A2X2系统,根据氢谱峰型和化学位移规律可以归属为H-10和H-11,但其三重峰并不标准.第4耦合系统是苯环上乙氧基δH 3.98 (2H, q, J = 7.0 Hz) 和δH 1.35 (3H, t, J = 7.0 Hz) 构成的A2X3系统,归属为H-20和H-21.第5耦合系统是苯乙基中两个亚甲基δH 3.65 (2H, t, J = 7.6 Hz) 和δH 3.27 (2H, t, J = 7.6 Hz) 构成的A2B2系统,直接与苯并咪唑环相连的CH2电子云密度更低,所以依次归属为H-14和H-15.根据尼秦类物质的结构通式(表1),尼秦类物质常用亚甲基连接的对位取代苯环,该CH2会受到苯并咪唑环和苯环的双重吸电子作用,所以1H NMR中常常会在δH 4.3附近呈现包含两个质子的单峰,因此该耦合系统的化学位移和峰型差异是区分苄基尼秦和苯乙基尼秦的重要判据.第6耦合系统是乙二胺氮上连接的两个乙基δH 3.36 (m, 4H) 和δH 1.39 (t, J = 7.3 Hz, 6H),通过化学位移规律可以很快速的归属为H-12a、H-12b和H-13a、H-13b,但氢谱的峰型变化却值得深入讨论.
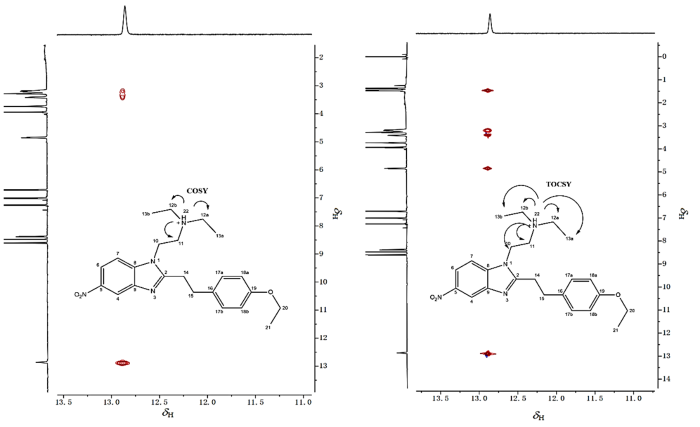
如果H-12a、H-12b与H-13a、H-13b形成的是简单A3B2自旋系统,那么按照n+1规律,H-13a和H-13b呈现三(即2+1)重峰是显而易见的,但H-12a和H-12b却并未按照n+1规律呈现出四(即3+1)重峰,反而呈现出近似八重峰,提示构成的自旋体系可能更为复杂.考虑到分子中存在活泼氢H-22,且化学位移较高呈现出酸性(图7),所以推测产生八重峰极有可能是叔胺成盐后存在的质子H-22参与了H-12a、H-12b与H-13a、H-13b的自旋耦合,使得相关氢实际形成的是A3B2X自旋系统(X为活泼氢),则此时H-12a和H-12b按照n+1规律,应形成八[即(3+1)×(1+1)]重峰.同时也能够合理解释H-10与H-11的三重峰并不标准的原因,即活泼氢H-22同样参与了H-10、H-11的自旋体系构建,H-22与H-10和H-11实际上形成的是AM2X2自旋系统,活泼氢H-22因化学交换速率快,耦合被平均化呈现单峰,但仍然影响了H-10、H-11的峰形使得其3重峰显著展宽并伴随峰高异常现象.为验证上述推断,对能够观察到活泼氢H-22的CDCl3溶剂体系使用1H-1H COSY和 1H-1H TOCSY(图9)观察H-22与H-10、H-11和H-12a、H-12b及H-13a、H-13b的耦合相关情况.1H-1H COSY显示H-22与H-11和H-12a、H-12b相关.1H-1H TOCSY显示成盐的活泼氢H-22与乙二胺片段上的H-10、H-11以及N,N-二乙基片段上的H-12a、H-12b和H-13a、H-13b相关,同属于一个自旋体系,上述结果充分证明了活泼氢H-22参与了第3耦合系统和第6耦合系统的构建.后续利用离子色谱检测发现样品含有高浓度(0.076 mg/mL)的氯离子,结合工艺流程图确定样品成盐,盐型为盐酸盐,进一步支持了上述推断.至此,氢谱已全部归属完毕,CD3OD溶剂体系下的 1H-1H COSY(图10)的相关耦合也同样支持了氢谱归属(表2).
图9
图9
苯乙基依托尼秦的1H-1H COSY和1H-1H TOCSY谱图(CDCl3, 11.0~13.5)
Fig. 9
1H-1H COSY and 1H-1H TOCSY spectrum of phenylethyl etonitazene (CDCl3, 11.0~13.5)
图10
图10
苯乙基依托尼秦的1H-1H COSY谱图(CD3OD)
Fig. 10
1H-1H COSY spectrum of phenylethyl etonitazene (CD3OD)
表2 苯乙基依托尼秦的 1H 和 13C NMR 归属(CD3OD)
Table 2
| Position | δC | DEPT | δH (J/Hz) | HSQC | 1H -1H COSY | 1H -1H TOCSY | 1H -13C HMBC |
|---|---|---|---|---|---|---|---|
| 2 | 160.2 | C | / | / | / | / | H-10, H-14, H-15 |
| 4 | 112.3 | CH | 8.68 (1H, d, 2.1) | + | / | H-6, H-7 | C-5, C-6, C-8, C-9 |
| 5 | 132.9 | C | / | / | / | / | H-4, H-7 |
| 6 | 122.5 | CH | 8.51 (1H, dd, 9.1, 2.1) | + | H-7 | H-4, H-7 | C-4, C-8, C-9 |
| 7 | 114.7 | CH | 8.29 (1H, d, 9.1) | + | H-6 | H-4, H-6 | C-4, C-5, C-9 |
| 8 | 136.8 | C | / | / | / | / | H-4, H-6, H-10 |
| 9 | 147.6 | C | / | / | / | / | H-4, H-6, H-7 |
| 10 | 40.9 | CH2 | 4.96 (2H, t, 8.3) | + | H-11 | H-11 | C-2, C-8, C-11 |
| 11 | 50.0 | CH2 | 3.43 (2H, t, 8.3) | + | H-10 | H-10 | C-10, C-12a, C-12b |
| 12a | 48.8 | CH2 | 3.36 (4H, m) | + | H-13a | H-13a, H-13b | C-11, C-12b, C-13a |
| 12b | 48.8 | CH2 | 3.36 (4H, m) | + | H-13b | H-13a, H-13b | C-11, C-12a, C-13b |
| 13a | 9.1 | CH3 | 1.39 (6H, t, 7.3) | + | H-12a | H-12a, H-12b | C-12a |
| 13b | 9.1 | CH3 | 1.39 (6H, t, 7.3) | + | H-12b | H-12a, H-12b | C-12b |
| 14 | 29.7 | CH2 | 3.65 (2H, t, 7.6) | + | H-15 | H-15 | C-2, C-15, C-16 |
| 15 | 33.0 | CH2 | 3.27 (2H, t, 7.6) | + | H-14 | H-14 | C-2, C-14, C-17a, C-17b |
| 16 | 131.6 | C | / | / | / | / | H-14, H-18a, H-18b |
| 17a | 130.7 | CH | 7.21 (2H, d, 8.6) | + | H-18a | H-18a, H-18b | C-15, C-17b, C-18a, C-19 |
| 17b | 130.7 | CH | 7.21 (2H, d, 8.6) | + | H-18b | H-18a, H-18b | C-15, C-17a, C-18b, C-19 |
| 18a | 116.0 | CH | 6.85 (2H, d, 8.6) | + | H-17a | H-17a, H-17b | C-16, C-18b, C-19 |
| 18b | 116.0 | CH | 6.85 (2H, d, 8.6) | + | H-17b | H-17a, H-17b | C-16, C-18a, C-19 |
| 19 | 159.7 | C | / | / | / | / | H-17a, H-17b, H-18a, H-18b, H-20 |
| 20 | 64.5 | CH2 | 3.98 (2H, q, 7.0) | + | H-21 | H-21 | C-19, C-21 |
| 21 | 15.1 | CH3 | 1.35 (3H, t, 7.0) | + | H-20 | H-20 | C-20 |
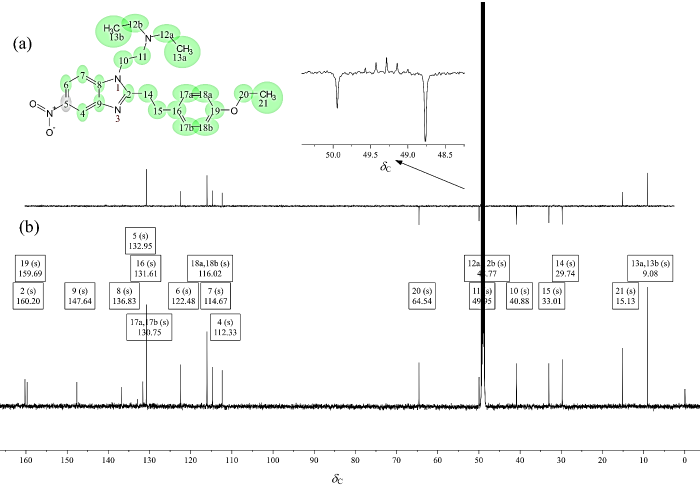
苯乙基依托尼秦的13C NMR[CD3OD,图11(b)]中有19组化合物碳峰,但根据结构式预期该分子应产生23组碳峰,实验观察到的信号组数少于理论预期,表明谱图中存在信号简并现象.这一现象源于分子结构中存在对称等价碳原子,导致部分化学位移相同或极其接近的碳信号在碳谱谱中重叠,从而在谱图上显示出更少的信号组数.重叠的谱峰信号强度会明显增加,通过谱图确定简并现象来自于分子中对位取代苯环中(C-17a、C-17b和C-18a、C-18b)以及N,N-二乙基(C-12a、C-12b和C-13a、C-13b)的对称性.根据DEPT-135谱[图11(a)]确认分子中含有7个CH2基团,印证了苯乙基依托尼秦是将依托尼秦的对位取代苄基替换成了苯乙基.根据氢谱结果,结合1H-13C HSQC(图12)可以实现所有非季碳的归属(表2),鉴于非季碳的归属已在表2中完整呈现,后文将重点讨论6个季碳原子的归属逻辑.
图11
图11
苯乙基依托尼秦的(a) DEPT-135谱和(b) 13C NMR谱(CD3OD)
Fig. 11
(a) DEPT-135 and (b) 13C NMR spectrum of phenylethyl etonitazene (CD3OD)
图12
图12
苯乙基依托尼秦的1H-13C HSQC谱
Fig. 12
1H-13C HSQC spectrum of phenylethyl etonitaze
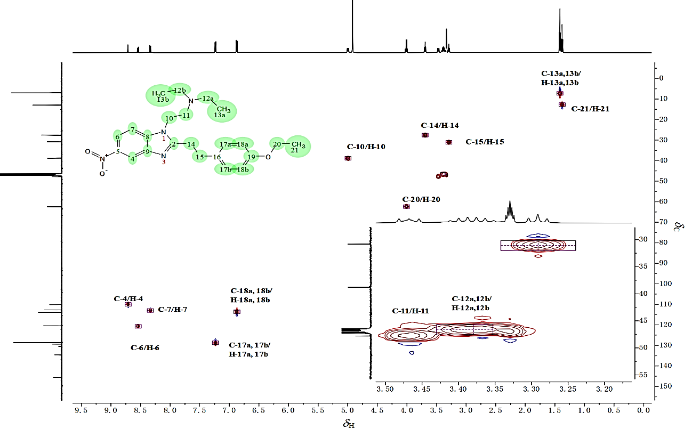
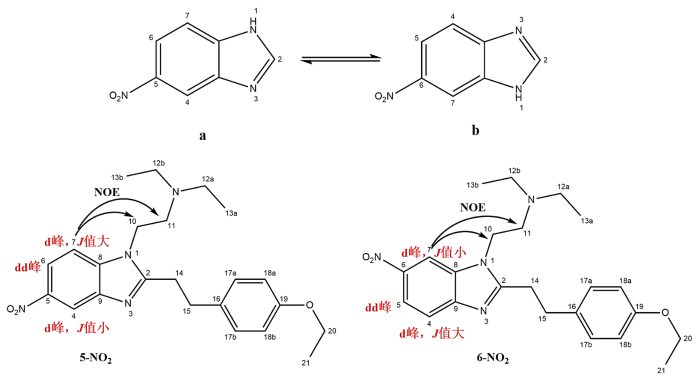
1H-13C HMBC(图13)指出,δ c 160.2与δH 4.96 (H-10)、δH 3.65 (H-14) 和δH 3.27 (H-15) 相关,表明δ c 160.2应归属为咪唑环的C-2.δ c 159.7与δH 7.21 (H-17a和H-17b)、δH 6.85 (H-18a和H-18b) 以及δH 3.98 (H-20) 相关,表明δ c 159.7应归属为苯环中与氧直接相连的C-19.δ c 147.6、δ c 136.8、δ c 132.9三个碳主要与苯并咪唑环的芳香氢相关,可以确定为苯环内季碳,继续归属前,需要先确认苯并咪唑环的构型,苯并咪唑环存在2种异构体,通过1H-1H NOESY(附件材料图S1)发现δH 8.29 (H-7) 与δH 4.96 (H-10) 和δH 3.43 (H-11) 相关,表明苯并咪唑环的构型为a而非b(图14,若为b型则H-7的耦合常数应小于H-4).
图13
图13
苯乙基依托尼秦的1H-13C HMBC局部放大图[CD3OD]. (a) δH: 6.7~9.1; (b) δH: 0.9~5.5
Fig. 13
The 1H-13C HMBC partial spectrum of phenylethyl etonitazen[CD3OD]. (a) δH: 6.7~9.1; (b) δH: 0.9~5.5
图14
最后详细分析苯并咪唑苯环内各季碳的耦合情况:δ c 147.6与δH 8.68 (H-4)、δH 8.51 (H-6) 和δH 8.29 (H-7) 都呈现出明显相关,故将其归属为C-9.δ c 136.8不仅与δH 8.68 (H-4) 和δH 8.51 (H-6) 相关,还与δH 4.96(H-10) 相关,将其归属为C-8.而δ c 132.9仅与δH 8.68 (H-4) 和δH 8.29 (H-7) 相关,将其归属为C-5.最后仅剩一个季碳δ c 131.6未归属,而δ c 131.6与δH 6.85 (H-18a和H-18b) 和δH 3.65 (H-14) 相关,将其归属为C-16.上述归属完全符合碳化学位移规律,同时1H-13C HMBC再次验证了氢谱归属.
3 结论
本文通过IR光谱、EI质谱和ESI高分辨质谱以及NMR波谱等方法对可疑缴获物中的苯乙基依托尼秦进行了全面的结构分析.IR图谱表明样品分子结构中含有硝基、叔胺、甲基、亚甲基、苯并咪唑环、芳香环、醚键等基团.EI质谱表明分子中可能含有二乙氨基和对位取代苯基片段.ESI高分辨质谱推测分子式与苯乙基依托尼秦实际分子式相符.NMR氢谱、碳谱及二维谱等均与结构相符.通过离子色谱,确证了缴获可疑物质为盐酸盐,为结晶性粉末.本文的研究结果对尼秦类化合物结构确证以及毒品司法鉴定具有重要参考意义.
利益冲突
无
附件材料附录
附件材料附录(可在《波谱学杂志》官网
图S1苯乙基依托尼秦的1H-1H NOESY谱图
参考文献
The challenge of new psychoactive substances. A technical update
[EB/OL]. [2024-06]. https://www.issup.net/files/2024-06/The_Challenge_of_NPS_A_technical_update_2024%20%281%29.pdf
Acute intoxications and fatalities associated with benzimidazole opioid (Nitazene Analog) use: a systematic review
[J].
DOI:10.1097/FTD.0000000000000970
PMID:35149665
[本文引用: 1]

Synthetic benzimidazole opioids (BO) are highly potent µ-opioid receptor agonists with heroin-like effects. Isotonitazene was first available in 2019 in the drug market, while new analogs have multiplied recently. The authors aimed to identify BO use trends and gather toxicological data from BO-related cases to assist in clinical and forensic investigations.A systematic literature search was conducted according to the PRISMA guidelines. PubMed and Scopus databases were accessed in October 2021 to identify scientific reports of BO-related intoxication and fatalities. Publication dates, case descriptions, symptoms, autopsy findings, and concentrations of BOs and metabolites in biological matrices were compiled.Data from 8 case reports with 93 fatalities involving isotonitazene (n = 65), metonitazene (n = 20), etonitazepyne (N-pyrrolidino etonitazene) (n = 8), flunitazene (n = 4), and/or butonitazene (n = 1), and 1 acute intoxication involving etonitazepyne were collected. Autopsy findings included pulmonary congestion/high lung weight (66%), cardiomegaly/high cardiac weight (39%), cerebral edema (22%), gastric contents in the airways (22%), and organ congestion (22%). Median peripheral blood concentrations were 1.7 ng/mL for isotonitazene (0.4-9.5 ng/mL, n = 13), 5.4 ng/mL for metonitazene (0.52-33 ng/mL, n = 17), 5.4 ng/mL for etonitazepyne (2.4-8.3 ng/mL, n = 2), 1.3 ng/mL for flunitazene (0.58-2.1 ng/mL, n = 2), and 3.2 ng/mL for butonitazene (n = 1). Central nervous system depressants were almost always co-administered.Isotonitazene was predominant in cases from 2019 to mid-2020 and was replaced by metonitazene after scheduling in the USA. Typical findings on opioid overdoses have been reported. Peripheral blood concentrations were consistent with a potency similar to that of fentanyl. These results must be interpreted carefully, considering the scarcity of reports on BO-related cases and drug co-exposures.Copyright © 2022 Wolters Kluwer Health, Inc. All rights reserved.
Synthesis, chemical characterization, and μ-opioid receptor activity assessment of the emerging group of “Nitazene” 2-benzylbenzimidazole synthetic opioids
[J].DOI:10.1021/acschemneuro.1c00064 URL [本文引用: 1]
Report on a novel emerging class of highly potent benzimidazole NPS opioids: chemical and in vitro functional characterization of isotonitazene
[J].
DOI:10.1002/dta.2738
PMID:31743619
[本文引用: 1]

This paper reports on the identification and full chemical characterization of isotonitazene (N,N-diethyl-2-[5-nitro-2-({4-[(propan-2-yl)oxy]phenyl}methyl)-1H-benzimidazol-1-yl]ethan-1-amine), a potent NPS opioid and the first member of the benzimidazole class of compounds to be available on online markets. Interestingly, this compound was sold under the name etonitazene, a structural analog. Identification of isotonitazene was performed by gas chromatography mass spectrometry (GC-MS) and liquid chromatography time-of-flight mass spectrometry (LC-QTOF-MS), the latter identifying an exact-mass m/z value of 411.2398. All chromatographic data indicated the presence of a single, highly pure compound. Confirmation of the specific benzimidazole regio-isomer was performed using H and C NMR spectroscopy, after which the chemical characterization was finalized by recording Fourier-transform (FT-IR) spectra. A live cell-based reporter assay to assess the in vitro biological activity at the μ-opioid receptor (MOR) revealed that isotonitazene has a high potency (EC of 11.1 nM) and efficacy (E 180% of that of hydromorphone), thus confirming that this substance is a strong opioid. Isotonitazene has not been previously detected, either in powder form, or in biological fluids. The high potency and efficacy of isotonitazene, combined with the fact that this compound was being sold undiluted, represents an imminent danger to anyone aiming to use this powder.© 2019 John Wiley & Sons, Ltd.
Federal Register. Drug enforcement administration: Schedules of controlled substances: Temporary placement of butonitazene, etodesnitazene, flunitazene, metodesnitazene, metonitazene, N-pyrrolidino etonitazene, and protonitazene in schedule I
[EB/OL]. [
Isotonitazene: fatal intoxication in three cases involving this unreported novel psychoactive substance in Switzerland
[J].DOI:10.1016/j.forsciint.2021.110686 URL [本文引用: 1]
DARK classics in chemical neuroscience: etonitazene and related benzimidazoles
[J].DOI:10.1021/acschemneuro.1c00037 URL [本文引用: 3]
Mass fragmentation characteristics of new psychoactive substances of nitazenes
[J].
尼秦类新精神活性物质的质谱特征研究
[J].
Characterization of mass spectrometry fragmentation patterns under electron‐activated dissociation (EAD) for rapid structure identification of nitazene analogs
[J].
Research progress of nitazenes new psychoactive substances and their testing methods
[J].
DOI:10.3969/j.issn.1671-2072.2024.04.003
[本文引用: 1]

New psychoactive substances (NPS), as the third generation of drugs after traditional drugs and synthetic drugs, are being abused globally, and have become a prominent issue of priority concern for countries around the world. Novel synthetic opioids are one of the most potentially harmful substances and have been abused increasingly, posing a serious threat to human health and public safety. As one class of novel synthetic opioids, the problem of manufacturing, smuggling and abuse of Nitazenes are becoming more and more seriously all over the world. In this paper, the basic structure, abuse and control, pharmacological and toxicological properties, metabolism and detection methods of Nitazenes were reviewed. Moreover, the development of detection technology of Nitazenes were briefly prospected, in order to provide reference for further study of Nitazenes.
尼秦类新精神活性物质及其检验方法研究进展
[J].
Metonitazene in the United States—Forensic toxicology assessment of a potent new synthetic opioid using liquid chromatography mass spectrometry
[J].DOI:10.1002/dta.v13.10 URL [本文引用: 1]
Pharmacologic characterization of substituted nitazenes at μ, κ, and Δ opioid receptors suggests high potential for toxicity
[J].
Benzimidazole derivatives and related heterocycles
[J].
Drug monographs
[EB/OL]. [
Drug monographs
[EB/OL]. [
NMR data analysis of manidipine hydrochloride
[J].
盐酸马尼地平的核磁共振数据解析
[J].盐酸马尼地平是第三代合成降压新药.本文利用一维、二维核磁共振(NMR)技术,包括1H NMR、13C NMR DEPT-135、1H-1H NOESY、1H-1H COSY、1H-13C HSQC和1H-13C HMBC,对其1H和13C NMR信号进行了全归属,进一步确证了其分子结构.同时,对其1H NMR和13C NMR谱中一些异常信号进行了讨论.
Structure characterization and analgesic activity of novel pyrazolo[3,4-d]pyrimidin-4-one derivatives
[J].
新型吡唑并[3,4-d]嘧啶-4-酮类衍生物的结构表征和镇痛活性
[J].本文以2-氰基-3-乙氧基丙烯酸乙酯与3,4-二甲基苯肼为原料,通过多步反应合成了三种新型吡唑并[3,4-d]嘧啶-4-酮类衍生物(A~C),通过核磁共振(NMR,包括1H NMR、13C NMR)和液相色谱-质谱联用(LC-MS)技术表征确证了其结构,并完整归属了三种化合物的1H NMR数据.对所合成的化合物1-(3,4-二甲基苯基)-6-甲基-5-[3-(哌啶-1-基)丙氧基]-1,5-二氢-4H-吡唑并[3,4-d]嘧啶-4-酮(A),通过小鼠脑部质谱成像和福尔马林实验进行了初步的体内镇痛活性评价,我们发现化合物A能透过血脑屏障,并产生显著且剂量依赖的镇痛活性.本研究为以吡唑并[3,4-d]嘧啶-4-酮为骨架的镇痛药物的研发提供了结构和体内活性的基础研究数据.
Structural elucidation of hybutimibe
[J].
海博麦布结构确证
[J].本文采用紫外吸收光谱、红外吸收光谱、质谱、核磁共振波谱(包含1H NMR、13C NMR、DEPT、1H-1H COSY、1H-1H NOESY、1H-13C HSQC和1H-13C HMBC)以及单晶衍射等方法对海博麦布进行结构分析,对其所有的1H NMR和13C NMR谱信号进行了归属,还通过差示扫描量热法、热重分析及粉末X-射线衍射分析对海博麦布晶型进行研究.