
Chinese Journal of Magnetic Resonance ›› 2025, Vol. 42 ›› Issue (3): 231-248.doi: 10.11938/cjmr20253149cstr: 32225.14.cjmr20253149
• Articles • Previous Articles Next Articles
SUI Meiju1,2, ZHANG Lei1,2, WANG Ruifang1,2, LUO Yingying1, LI Sha1, QIU Maosong1, XU Qiuyi1,2, CHEN Daiqin1,2, CHEN Shizhen1,2,3,*( ), ZHOU Xin1,2,3
), ZHOU Xin1,2,3
Received:2025-03-14
Published:2025-09-05
Online:2025-05-06
Contact:
* Tel: 027-87198631, E-mail: chenshizhen@apm.ac.cn.CLC Number:
SUI Meiju, ZHANG Lei, WANG Ruifang, LUO Yingying, LI Sha, QIU Maosong, XU Qiuyi, CHEN Daiqin, CHEN Shizhen, ZHOU Xin. MRI-traceable Nanoenzyme for Cascade Catalysis-enhanced Immunotherapy[J]. Chinese Journal of Magnetic Resonance, 2025, 42(3): 231-248.
Add to citation manager EndNote|Reference Manager|ProCite|BibTeX|RefWorks
Table 1
Experimental reagents and materials
| 试剂/材料名称 | 生产厂商 | 规格 | ||
|---|---|---|---|---|
| 氢氧化钠 | 中国医药集团有限公司 | 500 g | ||
| 浓盐酸 | 中国医药集团有限公司 | 500 mL | ||
| 四水合氯化锰 | 上海阿拉丁生化科技股份有限公司 | 500 g | ||
| 血红素 | 上海迈瑞尔生化科技有限公司 | 25 g | ||
| 谷胱甘肽 | 上海麦克林生化科技有限公司 | 25 g | ||
| 十二水合磷酸氢二钠 | 中国医药集团有限公司 | 500 g | ||
| 二水合磷酸二氢钠 | 中国医药集团有限公司 | 500 g | ||
| 5,5'-二硫代双(2-硝基苯甲酸)(DTNB) | 上海毕得医药科技有限公司 | 10 g | ||
| 3,3',5,5'-四甲基联苯胺(TMB) | 西格玛奥德里奇贸易有限公司 | 250 mg | ||
| 5,5-二甲基-1-吡咯啉-N-氧化物(DMPO) | 西格玛奥德里奇贸易有限公司 | 100 mg | ||
| 吲哚菁绿(ICG) | 上海麦克林生化科技股份有限公司 | 100 mg | ||
| 4',6-二脒基-2-苯基吲哚(DAPI)染色液 | 上海碧云天生物技术股份有限公司 | 50 mL | ||
| 2,7-二氯荧光素二乙酸酯(DCFH-DA) | 北京索莱宝科技有限公司 | 25 mg | ||
| 无菌PBS | HyClone | 500 mL | ||
| 胎牛血清 | 长沙赛尔博克斯生物科技有限公司 | 50 mL | ||
| RPMI 1640基础培养基 | Gibco Life Sciences公司 | 500 mL | ||
| DMEM基础培养基 | Gibco Life Sciences公司 | 500 mL | ||
| 青霉素-链霉素溶液 | Gibco Life Sciences公司 | 100 mL | ||
| 胰蛋白酶 | Gibco Life Sciences公司 | 100 mL | ||
| 抗体:CD80、CD86 | 赛默飞世尔科技有限公司 | 100 μg | ||
| 小鼠αPD-L1抗体 | BioXcell | 5 mg | ||
| IFN-β ELISA检测试剂盒 | 武汉贝茵莱生物科技有限公司 | 96 T | ||
| TNF-α ELISA检测试剂盒 | 武汉贝茵莱生物科技有限公司 | 96 T | ||
| 4T1、BEAS-2B细胞 | 中国科学院细胞库 | 1瓶 | ||
| Balb/c小鼠 | 湖北贝恩特生物科技有限公司 | 6周龄雌鼠 | ||
Table 2
Experimental instruments
| 仪器名称 | 生产厂商 | 型号 |
|---|---|---|
| pH计 | METTLER TOLEDO | FE20 |
| 磁力加热搅拌器 | IKA | HS 7 |
| 高速离心机 | Beckman Coulter | Advanti J-25 |
| 动态光散射仪 | Malvern | ZETASIZER Nano-ZS90 |
| 透射电子显微镜 | JEOL | JEM-2100 |
| 扫描电子显微镜 | Carl Zeiss AG | Zeiss SIGMA |
| 紫外-可见吸收光谱仪 | Thermo Fisher Scientific | Evolution 220 |
| 傅里叶变换红外吸光谱仪 | Thermo Fisher Scientific | Nicolet iS10 |
| X射线光电子能谱仪 | Thermo Fisher Scientific | ESCA Lab 250Xi |
| 电子顺磁共振波谱仪 | Bruker | Elexsys E580-10/12 |
| 808 nm激光器 | 北京镭志威 | LWIRL808 |
| 红外热成像仪 | 武汉红视热像科技 | HS160 |
| 溶氧仪 | 上海雷磁 | JPB-607A |
| 电感耦合等离子体质谱仪 | 德国耶拿分析仪器有限公司 | PQ-MS |
| 细胞培养箱 | Thermo Fisher Scientific | HERACELL 150i |
| 细胞计数仪 | 瑞沃德 | C100-SE/C100 |
| 无菌操作台 | Thermo Fisher Scientific | MCS ADVANTAGE |
| 共聚焦激光扫描显微镜 | Nikon | A1R/A1 |
| 流式细胞仪 | Beckman Coulter | CytoFLEX |
| 小动物麻醉机 | 瑞沃德 | R450 |
| 小动物光声成像仪 | iThera Medical GmbH | MOST inVision 256-TF |
| 小动物活体荧光成像仪 | Perkin Elmer | IVIS spectrum imaging system |
| 7T磁共振成像仪 | Bruker | BioSpec 70/20 USR |

Fig. 1
The characterization of MH NPs. (a) TEM and TEM mapping image of MH NPs; (b) Size distribution of MH NPs; (c) Zeta potential of MH NPs; (d) XPS spectra of Fe element in MH NPs; (e) XPS spectra of Mn element in MH NPs; (f) UV-vis absorption spectra of hemin and MH NPs; (g) FT-IR spectra of hemin and MH NPs


Fig. 2
The enzyme-like catalytic performance of MH NPs. (a) Schematic illustration of catalytic process of MH NPs; (b) UV-vis absorption spectra and photographs of DTNB after reaction of GSH with MH NPs solution at different concentrations (12.5, 25, 50, 100, and 200 μg·mL-1) ; (c) The generation of O2 over time after the reaction of H2O2 with MH NPs of different concentrations (0, 10, 20, 30, 40 and 50 μg·mL-1) ; (d) ESR spectra of H2O2 reacting with MH NPs (90 μL, 25 μg·mL-1), Fe2+ and H2O, respectively; (e) UV-vis absorption spectra of different reaction systems [TMB, TMB + H2O2, TMB + H2O2 + MH NPs (10 μL, 0.1 mg·mL-1)]; (f) UV-vis absorption spectra of reaction system [TMB + H2O2 + MH NPs (10 μL, 0.1 mg·mL-1)] at different times
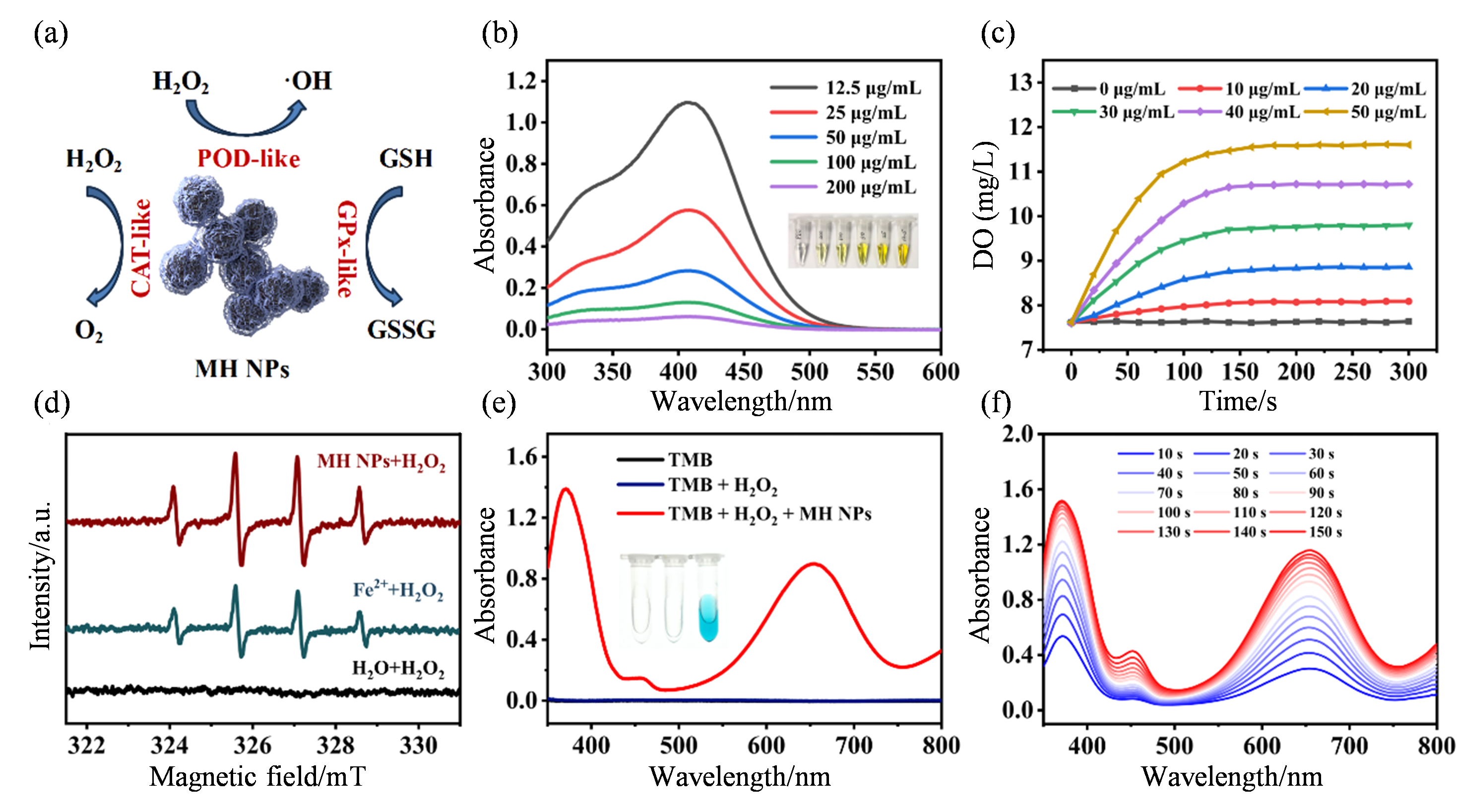
Fig. 3
The photothermal and photoacoustic performance of MH NPs. (a) The heating curve and infrared thermographs of MH NPs solution (500 μg·mL-1) and H2O under NIR laser irradiation (808 nm laser, 500 mW·cm-2); (b) Temperature change curves of MH NPs solutions with various concentrations irradiated by 808 nm laser (500 mW·cm-2); (c) Temperature change curves of MH NPs solution (500 μg·mL-1) irradiated by 808 nm laser of different power densities; (d) Calculation of the photothermal conversion efficiency of MH NPs; (e) Temperature change curves of MH NPs solutions under the cyclic irradiation of 808 nm laser (500 mW·cm-2); (f) Correlation of the photoacoustic signal intensity with the concentrations of MH NPs solutions and photoacoustic images of MH NPs solutions at different concentrations


Fig. 4
The T2-weighted MR imaging capability of MH NPs. (a) T2 MRI images of MH NPs at various pH conditions; (b) The linear relationship for the transverse relaxation rate of MH NPs as a function of Mn concentration under different pH conditions; (c) In vitro release curve of Mn2+ from MH NPs at various pH conditions (pH = 7.4, 6.5, and 5.5); (d) T2 MRI images of MH NPs after reaction with various concentrations of GSH; (e) The linear relationship for the transverse relaxation rate of MH NPs as a function of Mn concentration under different GSH conditions; (f) In vitro release curve of Mn2+ from MH NPs after reaction with various concentrations of GSH

Fig. 5
In vitro assessments of photothermal-enhanced anti-tumor catalytic therapy mediated by MH NPs. (a) Confocal fluorescence images of the cellular ROS level in 4T1 cells after different treatments (scale bar: 100 μm); (b) Semi-quantitative analysis of ROS level in 4T1 cells after different treatments; (c) Confocal fluorescence images of the cellular LPO level in 4T1 cells after different treatments (scale bar: 100 μm); (d) Semi-quantitative analysis of LPO level in 4T1 cells after different treatments; (e) Western blot assay of the STING signaling pathway-related protein expressed in 4T1 cells after different treatments; (f) Semi-quantitative analysis of the STING pathway-related protein expressed in 4T1 cells after different treatments; (g) The scheme of transwell system utilized to explore the maturation of dendritic cells induced by different treatments for 4T1 cells; (h) The expression level of IFN-β in the cell supernatant; (i) The expression level of TNF-α in the cell supernatant; (j) The level of mature dendritic cells measured by flow cytometry
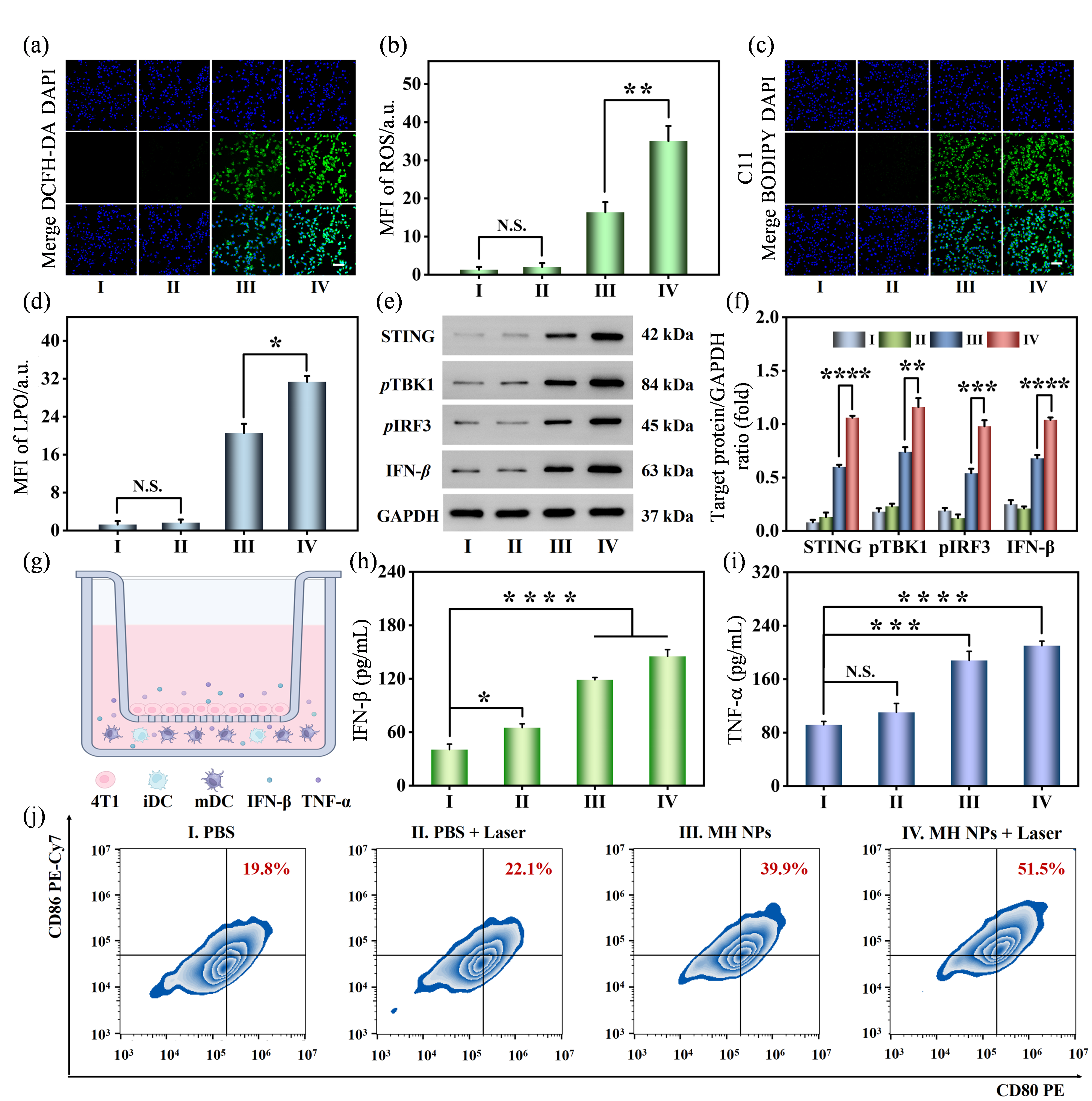

Fig. 6
In vivo biodistribution and MRI & PAI performance of MH NPs. (a) In vivo fluorescence images of 4T1 tumor-bearing mice at different time points after intravenous injection with MH@ICG NPs; (b) The fluorescence variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH@ICG NPs; (c) Fluorescence images of major organs of 4T1 tumor-bearing mice after intravenous injection with MH@ICG NPs for 72 h; (d) Semi-quantitive analysis of MH@ICG NPs in major organs of 4T1 tumor-bearing mice after intravenous injection with MH@ICG NPs for 72 h; (e) T2 MRI images of 4T1 tumor-bearing mice after intravenous injection with MH NPs at different time points; (f) Photoacoustic images of tumor sites in 4T1 tumor-bearing mice after intravenous injection with MH NPs at different time points; (g) The T2 MRI signal variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH NPs; (h) The photoacoustic signal variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH NPs
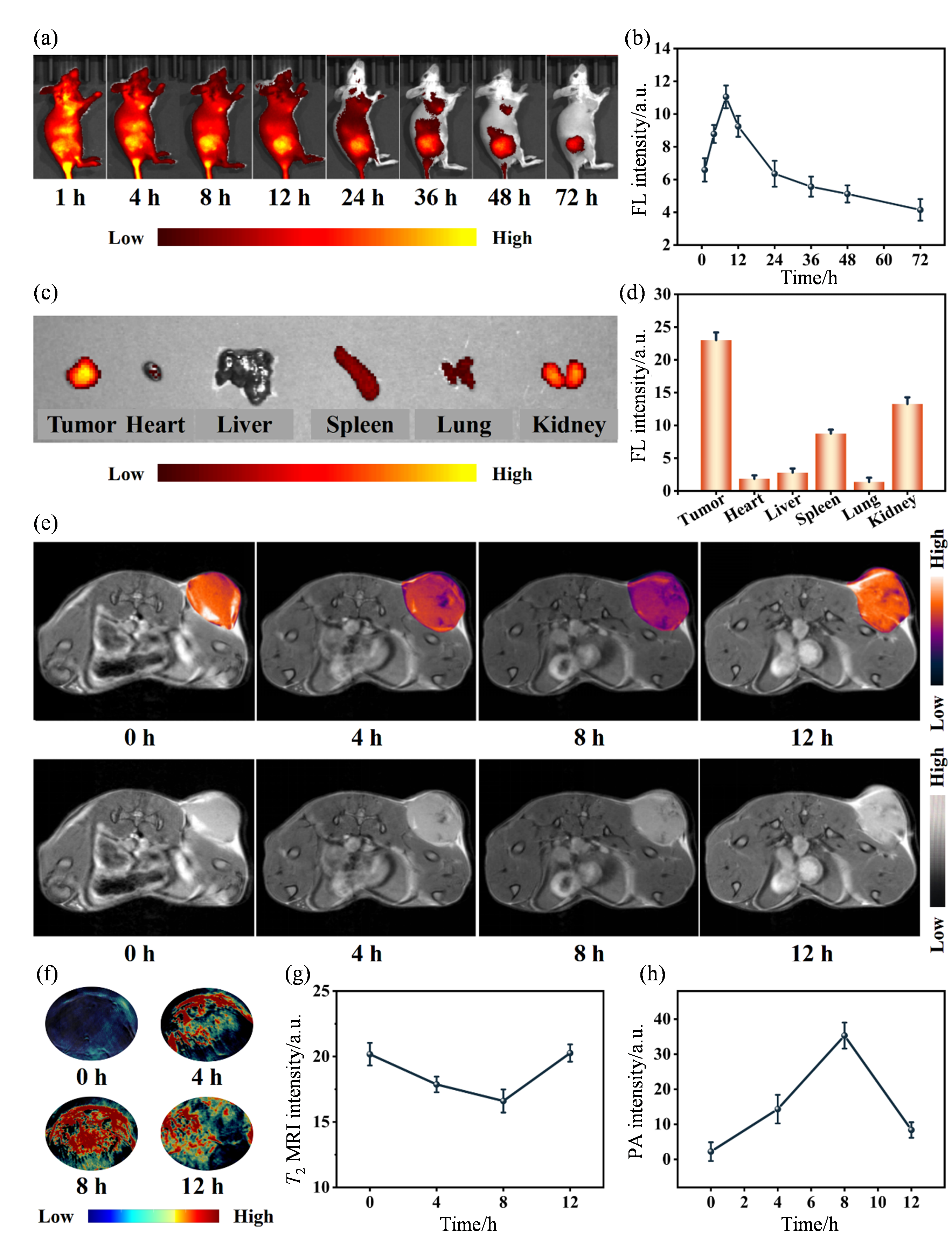

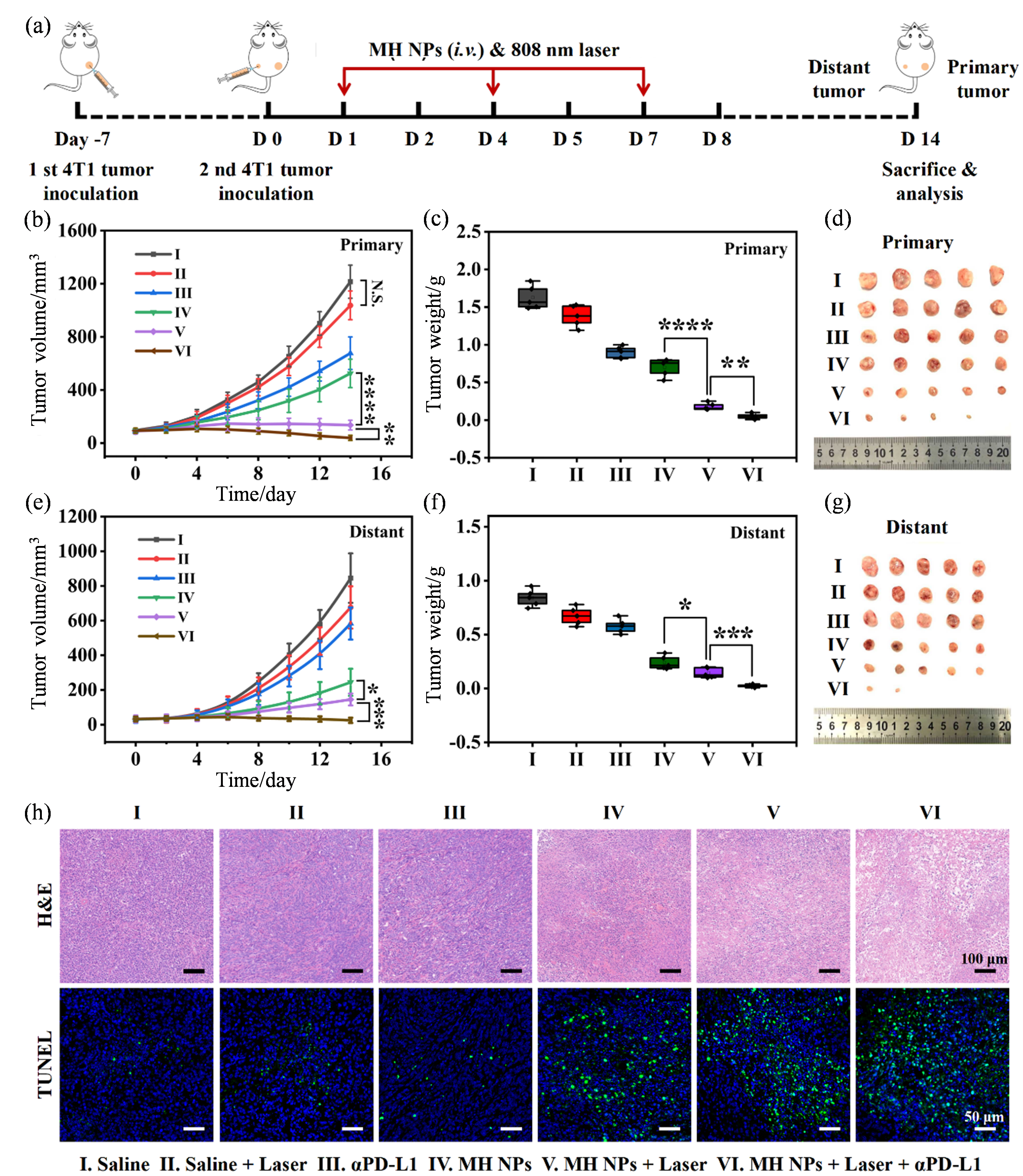
Fig. 7
Photothermal-enhanced MH NPs-mediated anti-tumor catalytic immunotherapy synergized with αPD-L1 against 4T1 tumor xenografts. (a) Schematic illustration of the photothermal-enhanced MH NPs-meidated anti-tumor catalytic immunotherapy synergized with αPD-L1; (b, e) Primary and distant tumor growth curves of the 4T1 tumor-bearing mice with different treatments; (c, f) The weight of primary and distant tumors in 4T1 tumor-bearing mice with different treatments; (d, g) Photographs of primary and distant tumors in 4T1 tumor-bearing mice with different treatments; (h) H&E and TUNEL staining histological analysis of primary tumor sections from 4T1 tumor-bearing mice with different treatments
| [1] | ANSELL S M, LESOKHIN A M, BORRELLO I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma[J]. N Engl J Med, 2014, 372(4): 311-319. |
| [2] | HODI F S, O'DAY S J, MCDERMOTT D F, et al. Improved survival with ipilimumab in patients with metastatic melanoma[J]. N Engl J Med, 2010, 363(8): 711-723. |
| [3] | MELLMAN I, COUKOS G, DRANOFF G. Cancer immunotherapy comes of age[J]. Nature, 2011, 480(7378): 480-489. |
| [4] |
VANNEMAN M, DRANOFF G. Combining immunotherapy and targeted therapies in cancer treatment[J]. Nat Rev Cancer, 2012, 12(4): 237-251.
doi: 10.1038/nrc3237 pmid: 22437869 |
| [5] | ALI S, KJEKEN R, NIEDERLAENDER C, et al. The European medicines agency review of kymriah (tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma[J]. Oncologist, 2020, 25(2): e321-e327. |
| [6] | SHI Y, LAMMERS T. Combining nanomedicine and immunotherapy[J]. Acc Chem Res, 2019, 52(6): 1543-1554. |
| [7] |
MUSETTI S, HUANG L. Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy[J]. ACS Nano, 2018, 12(12): 11740-11755.
doi: 10.1021/acsnano.8b05893 pmid: 30508378 |
| [8] | LARKIN J, CHIARION-SILENI V, GONZALEZ R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma[J]. N Engl J Med, 2015, 373(1): 23-34. |
| [9] |
FAN W P, YUNG B, HUANG P, et al. Nanotechnology for multimodal synergistic cancer therapy[J]. Chem Rev, 2017, 117(22): 13566-13638.
doi: 10.1021/acs.chemrev.7b00258 pmid: 29048884 |
| [10] | CHENG L, JIANG D, KAMKAEW A, et al. Renal-clearable PEGylated porphyrin nanoparticles for image-guided photodynamic cancer therapy[J]. Adv Funct Mater, 2017, 27(34): 1702928. |
| [11] | LI B, XIE X, CHEN Z, et al. Tumor inhibition achieved by targeting and regulating multiple key elements in EGFR signaling pathway using a self-assembled nanoprodrug[J]. Adv Funct Mater, 2018, 28(22): 1800692. |
| [12] | LI Y, XU N, ZHU W, et al. Nanoscale melittin@zeolitic imidazolate frameworks for enhanced anticancer activity and mechanism analysis[J]. ACS Appl Mater Interfaces, 2018, 10(27): 22974-22984. |
| [13] |
GONG Y, WANG P, CAO R, et al. Exudate absorbing and antimicrobial hydrogel integrated with multifunctional curcumin-loaded magnesium polyphenol network for facilitating burn wound healing[J]. ACS Nano, 2023, 17(22): 22355-22370.
doi: 10.1021/acsnano.3c04556 pmid: 37930078 |
| [14] | REN Z, SUN S, SUN R, et al. A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy[J]. Adv Mater, 2020, 32(6): e1906024. |
| [15] |
YANG W, DENG C, SHI X, et al. Structural and molecular fusion MRI nanoprobe for differential diagnosis of malignant tumors and follow-up chemodynamic therapy[J]. ACS Nano, 2023, 17(4): 4009-4022.
doi: 10.1021/acsnano.2c12874 pmid: 36757738 |
| [16] |
ZHANG Z, LI B, XIE L, et al. Metal-phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy[J]. ACS Nano, 2021, 15(10): 16934-16945.
doi: 10.1021/acsnano.1c08026 pmid: 34661387 |
| [17] | XIONG Y X, WANG W, DENG Q Y, et al. Mild photothermal therapy boosts nanomedicine antitumor efficacy by disrupting DNA mechanics damage repair pathways and modulating tumor[J]. Nano Today, 2023, 49: 101767. |
| [18] | ZHANG Y, TANG S Q, FENG X Y, et al. Tumor-targeting gene-photothermal synergistic therapies based on multifunctional polydopamine nanoparticles[J]. Chem Eng J, 2023, 457: 141315. |
| [19] |
ZHOU M X, WANG J X, PAN J X, et al. Nanovesicles loaded with a TGF-β receptor 1 inhibitor overcome immune resistance to potentiate cancer immunotherapy[J]. Nat Commun, 2023, 14(1): 3593.
doi: 10.1038/s41467-023-39035-x pmid: 37328484 |
| [20] | WANG B J, WANG T, JIANG T Z, et al. Circulating immunotherapy strategy based on pyroptosis and STING pathway: Mn-loaded paclitaxel prodrug nanoplatform against tumor progression and metastasis[J]. Biomaterials, 2024, 306: 122472. |
| [21] | CHEN C, SHEN X T, SHI S L, et al. Biomimetic Fe3+ metal-phenolic networks enable DNAzyme and Cas9 RNP delivery for synergistic tumor ferroptosis-immunotherapy[J]. Chem Eng J, 2024, 499: 156050. |
| [22] | INTAKHAD J, VACHIRAARUNWONG A, WONGPOOMCHAI R, et al. Ferric-tannic nanoparticles inhibit early-stage hepatocarcinogenesis by activating tumor immune responses in rats[J]. Adv Ther, 2024, 7(12): 2400348. |
| [23] | CHEN H Q, LIU L L, MA A Q, et al. Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer[J]. Biomaterials, 2021, 269: 120639. |
| [24] | LI Z F, WANG C M, DAI C, et al. Engineering dual catalytic nanomedicine for autophagy-augmented and ferroptosis-involved cancer nanotherapy[J]. Biomaterials, 2022, 287: 121668. |
| [25] | LIU J J, LIU S W, WU Y C, et al. Curcumin doped zeolitic imidazolate framework nanoplatforms as multifunctional nanocarriers for tumor chemo/immunotherapy[J]. Biomater Sci, 2022, 10(9): 2384-2393. |
| [26] | XIA Y X, JI X R, LI Q R. Research progress on application of immune checkpoint inhibitors combined with curcumin in tumor treatment[J]. Journal of Medical Forum, 2025, 46(7): 780-784. |
| 夏雨轩, 纪翛然, 李倩如. 免疫检查点抑制剂联合姜黄素在肿瘤治疗中的应用研究进展[J]. 医药论坛杂志, 2025, 46(7): 780-784. | |
| [27] | YAN H, HUANG S. Anti-tumor effect of curcumin on human colorectal cancer SW-620 cells[J]. Journal of Tianjin Medical University, 2024, 30(6): 479-484. |
| 阎晗, 黄珊. 姜黄素对人结直肠癌SW-620细胞抗肿瘤效果的研究[J]. 天津医科大学学报, 2024, 30(6): 479-484. | |
| [28] | CHEN L, LI Y Y, GAO F Y, et al. Study on the effect of dihydroartemisinin on ferroptosis in ovarian cancer cells by regulating SLC7A11/GPX4 signaling pathway[J]. Journal of Chinese Medicinal Materials, 2024, (12): 3103-3107. |
| 陈露, 李由由, 杲飞莹, 等. 双氢青蒿素通过调节SLC7A11/GPX4信号通路诱导卵巢癌细胞铁死亡[J]. 中药材, 2024, (12): 3103-3107. | |
| [29] | XU J R, JIA F J, CHEN J L, et al. Research of dihydroartemisinin on anti-colorectal cancer by regulating MAPK/PI3K/Akt signaling pathway[J]. Academic Journal of Shanghai University of Traditional Chinese Medicine, 2024, 38(2): 83-92. |
| 徐嘉若, 贾丰菁, 陈佳靓, 等. 双氢青蒿素通过调控MAPK/PI3K/Akt信号通路抗结直肠癌作用研究[J]. 上海中医药大学学报, 2024, 38(2): 83-92. | |
| [30] | HU X X, ZHAO Y, WANG Y, et al. Study on the nano-drug delivery system and anti-tumor mechanism of artemisinin and its derivatives[J]. Chemical Reagents, 2024, 46(7): 11-19. |
| 胡晓娴, 赵雨, 王赟, 等. 青蒿素及其衍生物纳米药物递送系统和抗肿瘤机制研究[J]. 化学试剂, 2024, 46(7): 11-19. | |
| [31] |
GUO Y J, DENG L, LI J, et al. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism[J]. ACS Nano, 2011, 5(2): 1282-1290.
doi: 10.1021/nn1029586 pmid: 21218851 |
| [32] | GOLUB E, FREEMAN R, WILLNER I. A hemin/G-quadruplex acts as an NADH oxidase and NADH peroxidase mimicking DNAzyme[J]. Angew Chem Int Ed Engl, 2011, 50(49): 11710-11714. |
| [33] |
YANG Y, ZHU W J, FENG L Z, et al. G-quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy[J]. Nano Lett, 2018, 18(11): 6867-6875.
doi: 10.1021/acs.nanolett.8b02732 pmid: 30303384 |
| [34] | LI K, XU K, HE Y, et al. Functionalized tumor-targeting nanosheets exhibiting Fe(II) overloading and GSH consumption for ferroptosis activation in liver tumor[J]. Small, 2021, 17(40): e2102046. |
| [35] |
LI X C, ZHANG X, SONG L, et al. Nanozyme as tumor energy homeostasis disruptor to augment cascade catalytic therapy[J]. ACS Nano, 2024, 18(51): 34656-34670.
doi: 10.1021/acsnano.4c09982 pmid: 39661982 |
| [36] | CHAO F R, CAO C L, XU Y, et al. Sprayable hydrogel for pH-responsive nanozyme-derived bacteria-infected wound healing[J]. ACS Appl Mater Interfaces, 2025, 17(4): 5921-5932. |
| [37] |
ELIA I, HAIGIS M C. Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism[J]. Nat Metab, 2021, 3(1): 21-32.
doi: 10.1038/s42255-020-00317-z pmid: 33398194 |
| [38] | JIN M Z, JIN W L. The updated landscape of tumor microenvironment and drug repurposing[J]. Signal Transduct Target Ther, 2020, 5(1): 166. |
| [39] | FAN Y, CHEN L, ZHENG Y, et al. Nanoparticle-based activatable MRI probes for disease imaging and monitoring[J]. Chem Biomed Imaging, 2023, 1(3): 192-204. |
| [40] |
WU F, DU Y Q, YANG J N, et al. Peroxidase-like active nanomedicine with dual glutathione depletion property to restore oxaliplatin chemosensitivity and promote programmed cell death[J]. ACS Nano, 2022, 16(3): 3647-3663.
doi: 10.1021/acsnano.1c06777 pmid: 35266697 |
| [41] | YANG B C, DAI Z C, ZHANG G R, et al. Ultrasmall ternary FePtMn nanocrystals with acidity-triggered dual-Ions release and hypoxia relief for multimodal synergistic chemodynamic/photodynamic/photothermal cancer therapy[J]. Adv Healthc Mater, 2020, 9(21): e1901634. |
| [42] |
ZHAO Z Y, DONG S M, LIU Y, et al. Tumor microenvironment-activable manganese-boosted catalytic immunotherapy combined with PD-1 checkpoint blockade[J]. ACS Nano, 2022, 16(12): 20400-20418.
doi: 10.1021/acsnano.2c06646 pmid: 36441901 |
| [43] | NEMETH T, PALLIER A, ÇELIK Ç, et al. Water-soluble Mn(III)-porphyrins with high relaxivity and photosensitization[J]. Chem Biomed Imaging, 2025, 3(1): 5-14. |
| [44] | ZENG Q B, GUO Q N, LUO Q, et al. Manganese-based contrast agents for MRI[J]. Chin J Magn Reson Imaging, 2014, 5(4): 315-320. |
| 曾庆斌, 郭茜旎, 罗晴, 等. 锰对比剂在MRI中的应用[J]. 磁共振成像, 2014, 5 (4): 315-320. | |
| [45] | ZHANG H X, YANG S P. Research progress of manganese(II)-based contrast agents in magnetic resonance imaging[J]. Journal of Shanghai Normal University (Natural Sciences), 2025, 54(1): 53-61. |
| 张何仙, 杨仕平. 锰(II)基造影剂在磁共振成像中的研究进展[J]. 上海师范大学学报(自然科学版), 2025, 54(1): 53-61. | |
| [46] |
CHEN L, DING C P, CHAI K J, et al. Nanohole-array induced metallic molybdenum selenide nanozyme for photoenhanced tumor-specific therapy[J]. ACS Nano, 2023, 17(18): 18148-18163.
doi: 10.1021/acsnano.3c05000 pmid: 37713431 |
| [47] | ZHU Y L, ZHAO R X, FENG L, et al. Dual nanozyme-driven PtSn bimetallic nanoclusters for metal-enhanced tumor photothermal and catalytic therapy[J]. ACS Nano, 2023, 17(7): 6833-6848. |
| [48] | PENG L, ZHAO A G, LI R K, et al. Self-propelled in situ polymerized nanoparticles activating the STING pathway for enhanced bladder cancer immunotherapy[J]. Adv Sci, 2025, 12: 2502750. |
| [1] | CHEN Xi, LIU Sijie, CAI Yue, CHENG Linlin, WANG Xuxia, KANG Yan, LIN Fuchun, LEI Hao. Effects of Seizure-inducing Doses Nicotine on Hippocampal Structure in Adolescent Female Rats [J]. Chinese Journal of Magnetic Resonance, 2025, 42(4): 345-354. |
| [2] | LI Yinghao, WANG Lihui, WANG Sucheng, ZHU Zhongqi, HUANG Changdong, LI Renfeng, CAO Kaiming, HU Haiyang, JIA Yiming, LIANG Songtao, YANG Guang, LU Qing, WANG Hongzhi. Study on Pancreas Automatic Segmentation, Regional Quantification, and Diabetes Assessment [J]. Chinese Journal of Magnetic Resonance, 2025, 42(4): 378-389. |
| [3] | MA Yingxue, ZHAO Yanqiang, YANG Xiaodong, JIANG Bin, TAO Cheng. Opportunities and Challenges of High-field and Ultra-high-field Magnetic Resonance Imaging in China [J]. Chinese Journal of Magnetic Resonance, 2025, 42(3): 334-344. |
| [4] | CHEN Qun, YANG Zijian, CHENG Xinyi, JIA Siyi, DU Xiaoxia, WANG Mengxing. Application of Magnetic Resonance Imaging Technology in Pediatric Exercise Intervention Research [J]. Chinese Journal of Magnetic Resonance, 2025, 42(2): 195-204. |
| [5] | PANG Qifan, WANG Zhichao, WU Yupeng, LI Jianqi. The Impact of K-Space Filling Strategy on Fat Artifacts in APT Imaging Based on FLASH Sequence [J]. Chinese Journal of Magnetic Resonance, 2024, 41(4): 443-453. |
| [6] | XU Zhenshun, YUAN Xiaohan, HUANG Ziheng, SHAO Chengwei, WU Jie, BIAN Yun. Multi-source Feature Classification Model of Pancreatic Mucinous and Serous Cystic Neoplasms Based on Deep Learning [J]. Chinese Journal of Magnetic Resonance, 2024, 41(1): 19-29. |
| [7] | LIU Ying, LIN Ling, YUAN Binhua, ZHANG Haowei. Research Progress of MRI Gradient Waveform Generator [J]. Chinese Journal of Magnetic Resonance, 2024, 41(1): 99-115. |
| [8] | LI Pan,FANG Delei,ZHANG Junxia,MA Debei. Magnetic Resonance Compatibility Analysis Method of Surgical Robotic System Based on Image Quality Evaluation [J]. Chinese Journal of Magnetic Resonance, 2023, 40(1): 79-91. |
| [9] |
De-gang TANG,Hong-chuang LI,Xiao-ling LIU,Lei SHI,Hai-dong LI,Chao-hui YE,Xin ZHOU.
A Simulation Study on the Effect of the High Permittivity Materials Geometrical Structure on the Transmit Field |
| [10] | Zhen-yu WANG, Ying-shan WANG, Jin-ling MAO, Wei-wei MA, Qing LU, Jie SHI, Hong-zhi WANG. Magnetic Resonance Images Segmentation of Synovium Based on Dense-UNet++ [J]. Chinese Journal of Magnetic Resonance, 2022, 39(2): 208-219. |
| [11] | Yan MA, Cang-ju XING, Liang XIAO. Knee Joint Image Segmentation and Model Construction Based on Cascaded Network [J]. Chinese Journal of Magnetic Resonance, 2022, 39(2): 184-195. |
| [12] | Jun LUO, Sheng-ping LIU, Xing YANG, Jia-sheng WANG, Ye LI. Design of a 5 T Non-magnetic Magnetic Resonance Radio Frequency Power Amplifier [J]. Chinese Journal of Magnetic Resonance, 2022, 39(2): 163-173. |
| [13] | Ju-min ZHANG,Shi-zhen CHEN,Xin ZHOU. Dual-modal MRI T1-T2 Contrast Agent Based on Dynamic Organic Gadolinium Nanoparticles [J]. Chinese Journal of Magnetic Resonance, 2022, 39(1): 11-19. |
| [14] | Zhi-chao WANG,Ji-lei ZHANG,Yu ZHAO,Ting HUA,Guang-yu TANG,Jian-qi LI. CEST Imaging of the Abdomen with Neural Network Fitting [J]. Chinese Journal of Magnetic Resonance, 2022, 39(1): 33-42. |
| [15] | Han-wei WANG,Hao WU,Jing TIAN,Jun-feng ZHANG,Peng ZHONG,Li-zhao CHEN,Shu-nan WANG. The Diagnostic Value of Quantitative Parameters of T2/FLAIR Mismatch Sign in Evaluating the Molecular Typing of Lower-grade Gliomas [J]. Chinese Journal of Magnetic Resonance, 2022, 39(1): 56-63. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||