引言
自免疫检查点抑制剂伊匹单抗被批准用于晚期黑色素瘤治疗以来,利用免疫系统清除肿瘤细胞为抗肿瘤治疗提供了全新思路[1
近年来,天然来源的生物活性化合物以其良好的生物安全性、抗氧化性、抗肿瘤活性和免疫调节特性而备受关注[10-
基于此,本文利用Mn2+和内源性血红素构建了集磁共振成像(MRI)与多种抗肿瘤治疗模式于一体的新型纳米酶-锰-血红素纳米配位聚合物(Mn-Hemin nanocoordination polymers,MH NPs).在成像方面,MH NPs可实现肿瘤靶向成像并实时追踪纳米酶在肿瘤内的分布,为个性化治疗方案的制定提供依据.在治疗方面,MH NPs继承了血红素卓越的类酶催化活性,在光热作用辅助下,可高效催化H2O2生成•OH并消耗谷胱甘肽(GSH),从而诱发过氧化损伤风暴,显著逆转ITME并激活抗肿瘤免疫应答.通过与免疫检查点抑制剂(Immune checkpoint inhibitor,ICI)αPD-L1联合治疗,MH NPs显著抑制了原发及远端转移肿瘤的生长.该研究为高效、安全的肿瘤诊疗一体化纳米平台提供了新思路,亦为优化免疫治疗策略提供了重要依据.
1 实验部分
1.1 实验试剂与材料
本研究所使用的实验试剂和材料如表1所示.
表1 实验试剂与材料
Table 1
| 试剂/材料名称 | 生产厂商 | 规格 | ||
|---|---|---|---|---|
| 氢氧化钠 | 中国医药集团有限公司 | 500 g | ||
| 浓盐酸 | 中国医药集团有限公司 | 500 mL | ||
| 四水合氯化锰 | 上海阿拉丁生化科技股份有限公司 | 500 g | ||
| 血红素 | 上海迈瑞尔生化科技有限公司 | 25 g | ||
| 谷胱甘肽 | 上海麦克林生化科技有限公司 | 25 g | ||
| 十二水合磷酸氢二钠 | 中国医药集团有限公司 | 500 g | ||
| 二水合磷酸二氢钠 | 中国医药集团有限公司 | 500 g | ||
| 5,5'-二硫代双(2-硝基苯甲酸)(DTNB) | 上海毕得医药科技有限公司 | 10 g | ||
| 3,3',5,5'-四甲基联苯胺(TMB) | 西格玛奥德里奇贸易有限公司 | 250 mg | ||
| 5,5-二甲基-1-吡咯啉-N-氧化物(DMPO) | 西格玛奥德里奇贸易有限公司 | 100 mg | ||
| 吲哚菁绿(ICG) | 上海麦克林生化科技股份有限公司 | 100 mg | ||
| 4',6-二脒基-2-苯基吲哚(DAPI)染色液 | 上海碧云天生物技术股份有限公司 | 50 mL | ||
| 2,7-二氯荧光素二乙酸酯(DCFH-DA) | 北京索莱宝科技有限公司 | 25 mg | ||
| 无菌PBS | HyClone | 500 mL | ||
| 胎牛血清 | 长沙赛尔博克斯生物科技有限公司 | 50 mL | ||
| RPMI 1640基础培养基 | Gibco Life Sciences公司 | 500 mL | ||
| DMEM基础培养基 | Gibco Life Sciences公司 | 500 mL | ||
| 青霉素-链霉素溶液 | Gibco Life Sciences公司 | 100 mL | ||
| 胰蛋白酶 | Gibco Life Sciences公司 | 100 mL | ||
| 抗体:CD80、CD86 | 赛默飞世尔科技有限公司 | 100 μg | ||
| 小鼠αPD-L1抗体 | BioXcell | 5 mg | ||
| IFN-β ELISA检测试剂盒 | 武汉贝茵莱生物科技有限公司 | 96 T | ||
| TNF-α ELISA检测试剂盒 | 武汉贝茵莱生物科技有限公司 | 96 T | ||
| 4T1、BEAS-2B细胞 | 中国科学院细胞库 | 1瓶 | ||
| Balb/c小鼠 | 湖北贝恩特生物科技有限公司 | 6周龄雌鼠 | ||
1.2 实验仪器
本研究所使用的实验仪器如表2所示.
表2 实验仪器
Table 2
| 仪器名称 | 生产厂商 | 型号 |
|---|---|---|
| pH计 | METTLER TOLEDO | FE20 |
| 磁力加热搅拌器 | IKA | HS 7 |
| 高速离心机 | Beckman Coulter | Advanti J-25 |
| 动态光散射仪 | Malvern | ZETASIZER Nano-ZS90 |
| 透射电子显微镜 | JEOL | JEM-2100 |
| 扫描电子显微镜 | Carl Zeiss AG | Zeiss SIGMA |
| 紫外-可见吸收光谱仪 | Thermo Fisher Scientific | Evolution 220 |
| 傅里叶变换红外吸光谱仪 | Thermo Fisher Scientific | Nicolet iS10 |
| X射线光电子能谱仪 | Thermo Fisher Scientific | ESCA Lab 250Xi |
| 电子顺磁共振波谱仪 | Bruker | Elexsys E580-10/12 |
| 808 nm激光器 | 北京镭志威 | LWIRL808 |
| 红外热成像仪 | 武汉红视热像科技 | HS160 |
| 溶氧仪 | 上海雷磁 | JPB-607A |
| 电感耦合等离子体质谱仪 | 德国耶拿分析仪器有限公司 | PQ-MS |
| 细胞培养箱 | Thermo Fisher Scientific | HERACELL 150i |
| 细胞计数仪 | 瑞沃德 | C100-SE/C100 |
| 无菌操作台 | Thermo Fisher Scientific | MCS ADVANTAGE |
| 共聚焦激光扫描显微镜 | Nikon | A1R/A1 |
| 流式细胞仪 | Beckman Coulter | CytoFLEX |
| 小动物麻醉机 | 瑞沃德 | R450 |
| 小动物光声成像仪 | iThera Medical GmbH | MOST inVision 256-TF |
| 小动物活体荧光成像仪 | Perkin Elmer | IVIS spectrum imaging system |
| 7T磁共振成像仪 | Bruker | BioSpec 70/20 USR |
1.3 MH NPs的合成与表征
1.3.1 合成
称取8.1 mg血红素溶于25 mL 0.1 mol·L-1氢氧化钠溶液,制备得到0.5 mmol·L-1血红素溶液,用盐酸将血红素溶液pH调至7.另称取395.8 mg四水合氯化锰溶于50 mL去离子水,制备得到40 mmol·L-1氯化锰溶液.在室温搅拌条件下,向血红素溶液中逐滴滴加氯化锰溶液,并使反应体系pH最终维持在7.继续反应1 h后,将反应溶液转移至高压反应釜中,在120 ℃下反应3 h.反应结束后,自然冷却至室温,收集深棕色产物并用去离子水反复洗涤,直至上清液澄清.最后,将产物分散于超纯水中,在-4 ℃条件下保存备用.
1.3.2 表征
使用透射电子显微镜(TEM)观察MH NPs的形貌、尺寸和元素组成.使用纳米激光粒度仪测定MH NPs的水合粒径和ζ电势.使用电感耦合等离子体质谱仪(ICP-MS)测定MH NPs中锰离子的浓度.使用X射线光电子能谱仪采集X射线光电子能谱(X-ray photoelectron spectra, XPS)以分析MH NPs中锰元素和铁元素的价态.分别使用紫外-可见分光光度计和傅里叶变换红外吸收光谱仪采集MH NPs的紫外-可见吸收光谱(Ultraviolet-visible absorption spectra, UV-vis spectra)和傅里叶变换红外吸收光谱(Fourier transform infrared absorption spectra, FT-IR spectra).
1.4 MH NPs的体外性能评估
1.4.1 类酶催化活性
以5,5'-二硫代双(2-硝基苯甲酸)(DTNB)为底物,通过其显色变化评估MH NPs的类谷胱甘肽过氧化物酶(GPx)活性.通过3,3',5,5'-四甲基联苯胺(TMB)显色反应和电子自旋共振(ESR)实验检验MH NPs的类过氧化物酶(POD)活性.使用溶氧仪检测MH NPs催化H2O2产生的氧气(O2)的量,评估MH NPs的类过氧化氢酶(CAT)活性.
1.4.2 光热性能
分别将不同浓度(200、400、600、800和1 000 μg·mL-1)的MH NPs溶液置于808 nm激光(500 mW·cm-2)下20 min,使用红外热成像仪实时监测溶液温度变化.分别使用不同功率808 nm激光(200、400、600、800和1 000 mW·cm-2)照射MH NPs溶液浓度(500 μg·mL-1)20 min,实时监测温度变化情况.分别对不同浓度(200、400、800和1 000 μg·mL-1)的MH NPs溶液进行多次激光照射-冷却循环,单次照射-冷却的监测时长分别为10 min,根据温度变化的一致性情况评估MH NPs的光热稳定性.使用功率为500 mW·cm-2的808 nm激光分别照射纯水和MH NPs溶液(质量浓度为1 mg·mL-1)10 min,实时采集MH NPs溶液的红外热像图并记录温度变化情况.
1.4.3 光声成像性能
使用MSOT inVision 256-TF小动物成像系统,在808 nm激发波长(Laser power平均值为44 mJ)下采集不同浓度(0、0.2、0.3、0.4、0.6、0.8、1.0、1.5、2.0、2.5和3.0 mg·mL-1)MH NPs溶液的光声信号,并通过软件对信号强度进行定量分析,以评估其光声成像性能.
1.4.4 磁共振成像性能
将MH NPs分散于不同pH的磷酸盐缓冲溶液(PBS)中,制备得到锰浓度分别为12、24、48、96、192和384 μmol·L-1的MH NPs溶液.使用7T磁共振成像仪采集各溶液的T1和T2 MRI图像,并记录纵向弛豫时间(T1)和横向弛豫时间(T2),评估不同pH条件下MH NPs的MRI成像性能.
将MH NPs与不同浓度GSH溶液(按照所需GSH溶液的浓度和体积,计算所需GSH药品的质量并使用天平准确称取量,溶于对应体积的去离子水中)混合,制备得到锰的终浓度分别为12、24、48、96、192和384 μmol·L-1而GSH的终浓度分别为1.25、2.5、5和10 mmol·L-1的MH NPs溶液.使用7T磁共振成像仪采集各溶液的T1和T2 MRI图像,并记录其T1和T2值,评估不同GSH浓度下MH NPs的MRI成像性能.
1.5 MH NPs的细胞实验
1.5.1 细胞毒性实验
使用细胞计数试剂盒-8(CCK-8)检测不同处理方式下MH NPs对人正常肺上皮细胞(BEAS-2B)和小鼠乳腺癌细胞(4T1)的细胞毒性.以BEAS-2B细胞为例,具体操作如下:
(1)将对数生长期的BEAS-2B细胞以5×103个/孔的密度接种于两个96孔板中,培养过夜.
(2)分别向两个96孔板中加入不同MH NPs浓度(分散介质为细胞完全培养基,0、12.5、25、50、100、150和200 μg·mL-1)的DMEM完全培养基,每孔200 μL.
(3)共同孵育6 h后,移除孵育溶液并用PBS清洗细胞3次,补加200 μL完全培养基,继续培养24 h.其中一个96孔板置于808 nm激光(1 000 mW·cm-2)下照射10 min后继续培养24 h.
(4)培养结束后,移除培养基并用PBS清洗细胞3次.向每孔加入100 μL的DMEM培养基和10 μL的CCK-8试剂,孵育1 h.
(5)使用酶标仪测定450 nm处的吸光度,并按(1)式计算细胞存活率C:
其中,
1.5.2 活性氧水平评估
为验证MH NPs的光热作用是否可以促进肿瘤细胞中活性氧(ROS)的生成,设置PBS、PBS + 激光(Laser)、MH NPs和MH NPs + Laser四个分组,使用DCFH-DA荧光探针检测各组4T1肿瘤细胞的ROS生成情况.
1.5.3 脂质过氧化水平评估
为验证MH NPs是否可通过催化生成过量的ROS诱发脂质过氧化(细胞铁死亡的标志之一),设置PBS、PBS + Laser、MH NPs和MH NPs + Laser四组,使用C11-BODIPY581/591荧光探针检测各组4T1肿瘤细胞的脂质过氧化水平.
1.5.4 铁死亡评估
为验证MH NPs是否能诱导肿瘤细胞发生铁死亡,设置PBS、PBS + Laser、MH NPs和MH NPs + Laser四组,通过Western Blot实验评估不同组别4T1肿瘤细胞内铁死亡相关蛋白(Glutathione Peroxidase 4,GPX4)的表达情况.
1.5.5 STING通路激活
为验证MH NPs是否能激活STING(Stimulator of interferon gene)信号通路,设置PBS、PBS + Laser、MH NPs和MH NPs + Laser四组,通过Western Blot实验评估不同组别4T1肿瘤细胞内STING信号通路相关蛋白(STING、pTBK1、pIRF3和IFN-β)的表达水平.
1.5.6 IFN-β、TNF-α水平评估
为进一步验证MH NPs是否具备激活4T1细胞STING通路的能力,设置PBS、PBS + Laser、MH NPs和MH NPs + Laser四组,通过Transwell实验和IFN-β、TNF-α细胞因子ELISA试剂盒评估不同组别细胞上清溶液中IFN-β和TNF-α的水平.
1.5.7 树突状细胞熟化
为验证MH NPs是否能通过激活STING信号通路促进树突状细胞成熟,设置PBS、PBS + Laser、MH NPs和MH NPs + Laser四组,使用流式细胞术评估不同处理条件下树突状细胞的成熟情况.具体操作如下:
(1)分别将对数生长期的4T1细胞和未成熟树突状细胞接种于transwell系统的上腔室和下腔室,并在培养箱(37 ℃,5% CO2)中培养过夜.
(2)细胞贴壁后,分别向上腔室加入PBS或含MH NPs(分散介质为1 640完全培养基,100 μg·mL-1)的培养基,对于激光照射组,共孵育4 h后,使用808 nm激光(1 000 mW·cm-2)照射10 min.
(3)继续孵育24 h后,使用PBS清洗下腔室中的树突状细胞并使用anti-CD45 APC抗体、anti-CD11c FITC抗体、anti-CD86 PE-Cy7抗体和anti-CD80 PE抗体进行染色.染色结束后,使用PBS清洗细胞3次,并用流式细胞仪检测不同组别树突状细胞的熟化情况.
1.6 MH NPs的活体成像实验
1.6.1 4T1皮下肿瘤造模
所有动物实验均按照中国科学院精密测量科学与技术创新研究院动物伦理审查委员会提供并批准的指南(APM21013T)进行.收集对数生长期的4T1细胞并用PBS重悬得到浓度为5×106个/mL的细胞悬液.选取6周龄雌性Balb/c小鼠为造模对象,向小鼠右后腿皮下注射200 μL细胞悬液,当肿瘤体积达到实验要求后,开展活体相关实验.
1.6.2 MH NPs的生物代谢分布
使用吲哚菁绿(ICG)标记MH NPs,得到MH@ICG NPs.向5只荷瘤小鼠的尾静脉内分别注射100 μL的MH@ICG NPs溶液(分散介质为生理盐水,2 mg·mL-1),在注射后不同时间点(1、4、8、12、24、36、48和72 h),使用IVIS小动物荧光成像仪采集小鼠的荧光图像并定量分析肿瘤部位的荧光强度.72 h后对小鼠实施安乐死,取主要脏器(心、肝、脾、肺和肾)进行离体荧光成像,并分析MH@ICG NPs在体内的生物分布情况.
1.6.3 活体磁共振及光声成像
为评估MH NPs对肿瘤的T2磁共振成像和光声成像(PAI)效果,随机选取5只荷瘤小鼠并分别使用7T磁共振成像仪和MSOT inVision 256-TF小动物光声成像仪对其进行成像.具体操作如下:
向荷瘤小鼠的尾静脉内注射200 μL现配的MH NPs溶液(分散介质为生理盐水,2 mg·mL-1),在注射后不同时间点(0、4、8和12 h),采集荷瘤小鼠的T2 MRI图像和光声图像.其中,7T磁共振成像仪的采集参数为:Turbo RARE-T2序列,重复时间(Time of repetition, TR)= 2 500 ms, 回波时间(Time of echo, TE)= 25 ms,平均次数(Number of Average)= 4,RARE因子(RARE factor)= 1,矩阵大小(Matrix size)= 256 × 256,层厚(Slice thickness)= 1 mm;MSOT inVision 256-TF小动物光声成像仪的激发波长为808 nm.
1.7 MH NPs的抗肿瘤治疗实验
使用双侧肿瘤小鼠模型评估MH NPs协同αPD-L1的抗肿瘤催化免疫治疗效果,具体操作如下:
(1)收集对数生长期的4T1细胞,用PBS重悬使细胞悬液的浓度为5×106个/mL.
(2)如治疗示意图所示,分别于治疗开始之前8天和1天,向小鼠的右后腿和左后腿皮下注射200 μL细胞悬液,形成双侧肿瘤.右后腿肿瘤视为“原发肿瘤”,左后腿肿瘤视为“远端转移肿瘤”.
(3)将30只造模成功的荷瘤小鼠随机分为6组:I. Saline; II. Saline + Laser; III. αPD-L1; IV. MH NPs; V. MH NPs + Laser; VI. MH NPs + Laser + αPD-L1.
(4)在第0、3、6天,分别向I组和II组荷瘤小鼠尾静脉注射200 μL生理盐水,向IV组、V组和VI组荷瘤小鼠尾静脉注射200 μL MH NPs溶液(分散介质为生理盐水,2 mg·mL-1).对于II、V和VI组,尾静脉给药8 h后,使用808 nm激光(800 mW·cm-2)照射肿瘤部位10 min.在第1、4、7天向III组和VI组荷瘤小鼠尾静脉注射αPD-L1,每只小鼠的注射剂量为25 µg.
(5)在治疗的第14天,对各组小鼠进行安乐死处理,收集每只小鼠的原发和远端肿瘤并进行苏木精-伊红(H&E)和TUNEL染色,收集主要脏器(心、肝、脾,肺和肾)进行H&E染色.
(6)治疗期间,每两天测量一次原发肿瘤和远端转移肿瘤的体积并记录小鼠的体重.肿瘤体积按(2)式计算:
其中,V代表肿瘤的体积,L代表肿瘤的长度,W代表肿瘤的宽度.
1.8 统计分析
所有实验结果以“平均值±标准偏差(Standard deviation,SD)”表示,并采用t检验(Student's t test)进行统计学分析.结果可接受的显著性水平为*p < 0.05,**p < 0.01,***p < 0.001,****p < 0.0001,N.S.代表无显著性差异.
2 结果与讨论
2.1 MH NPs表征
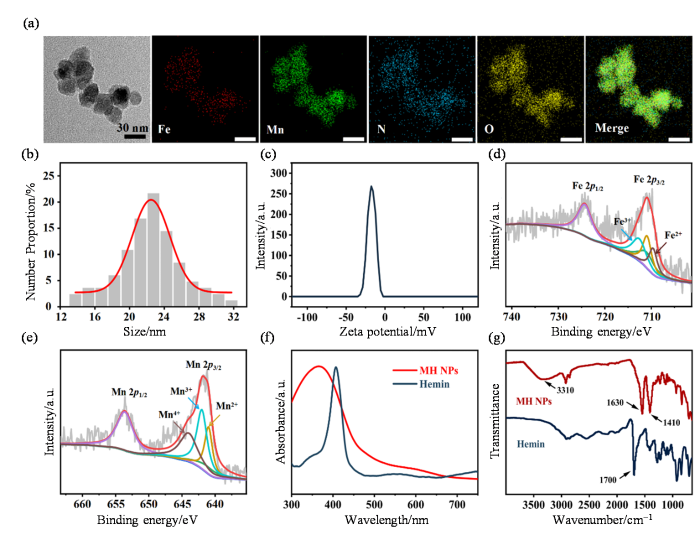
MH NPs通过锰离子与血红素间的配位聚合作用自组装形成,锰离子作为金属离子配位中心,血红素化学结构中两个不对称的羧基充当桥联配体,在中性pH条件下,二者配位并最终形成MH NPs.TEM结果显示,MH NPs呈不规则球形状,平均粒径约为22.4 nm[图1(a)、(b)].纳米激光粒度仪的测定结果显示,MH NPs的ζ电势为-17.5 mV[图1(c)].TEM Mapping结果显示,MH NPs中同时存在锰、铁、碳、氮和氧元素,证实了锰离子与血红素的成功配位[图1(a)和附件材料图S1].XPS结果显示,Fe 2p3/2在709.6 eV、710.9 eV和713.8 eV处的三个显著峰证实了MH NPs中Fe2+和Fe3+的存在[图1(d)];Mn 2p3/2在641 eV、642 eV和644 eV处的三个显著峰则分别归属于Mn2+、Mn3+和Mn4+[图1(e)].紫外-可见吸收光谱显示,与锰离子配位后,血红素的Soret带吸收峰变宽并由405 nm蓝移至398 nm[图1(f)],吸收峰变宽表明了MH NPs中血红素的超分子特性,而吸收峰蓝移则表明锰离子与血红素发生了配位作用.此外,红外吸收光谱结果显示,MH NPs在3 310 cm-1处的N-H特征吸收峰依然存在,而血红素在1 700 cm-1处的羧基特征吸收峰消失,转而在1 630 cm-1和1 410 cm-1处出现两个新的羧基吸收峰[图1(g)],这表明锰离子并未与血红素卟啉环上的氨基配位,而是与环外的羧基配位形成MH NPs.以上结果充分证明锰离子成功与血红素的羧基发生配位,实现了自组装并在产物中保留了Fe(II)、Fe(III)、Mn(II)、Mn(III)和Mn(IV)等多价态金属元素,为MH NPs后续展现多种类酶催化活性奠定了基础.
图1
图1
MH NPs的表征. (a) MH NPs的TEM和TEM mapping图;(b) MH NPs的粒径分布;(c) MH NPs的ζ电势;(d) MH NPs中Fe元素的X射线光电子能谱;(e) MH NPs中Mn元素的X射线光电子能谱;(f)血红素(Hemin)和MH NPs的紫外-可见吸收光谱;(g)血红素(Hemin)和MH NPs的傅里叶变换红外吸收光谱
Fig. 1
The characterization of MH NPs. (a) TEM and TEM mapping image of MH NPs; (b) Size distribution of MH NPs; (c) Zeta potential of MH NPs; (d) XPS spectra of Fe element in MH NPs; (e) XPS spectra of Mn element in MH NPs; (f) UV-vis absorption spectra of hemin and MH NPs; (g) FT-IR spectra of hemin and MH NPs
2.2 MH NPs体外性能评估
2.2.1 类酶催化性能
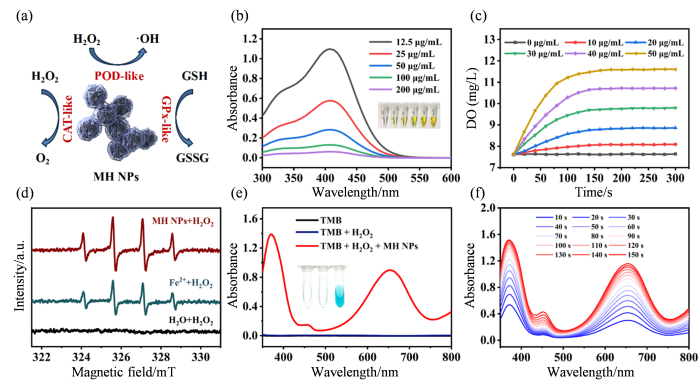
作为细胞内最重要的抗氧化剂之一,GSH能维持氧化还原稳态并保护细胞免受ROS损伤.然而,肿瘤细胞中高水平的GSH严重削弱了ROS介导的抗肿瘤治疗效果[35].因此,通过耗竭肿瘤细胞内GSH以瓦解其抗氧化防御,对提升ROS介导的抗肿瘤治疗疗效具有重要意义.本研究中,MH NPs中高价态Fe(III)、Mn(III)和Mn(IV)赋予了其类GPx活性.Ellman实验结果显示,MH NPs能有效消耗GSH,并随浓度增加而呈现更显著的耗竭效应[图2(b)].进一步研究发现,MH NPs还具有催化H2O2生成O2的CAT活性,且O2的生成速率随MH NPs浓度的增加而增大[图2(c)].以上结果表明,MH NPs可通过耗竭GSH和生成氧气改善ITME,从而助力ROS介导的抗肿瘤治疗.此外,本研究进一步通过ESR实验和TMB显色反应验证MH NPs是否继承了血红素的POD活性.ESR实验结果显示,与Fe2+和H2O2的混合溶液类似,MH NPs和H2O2的混合溶液同样检测到•OH的ESR特征信号[图2(d)],这表明MH NPs能通过类似于芬顿反应的方式催化H2O2产生•OH[36].以TMB为•OH指示剂的显色反应进一步证实了这一结论:在pH 6.5条件下,将MH NPs加入TMB和H2O2的混合溶液后,溶液于3 min内迅速变蓝,并在370 nm和652 nm处出现两个氧化型TMB的特征吸收峰[图2(e)],这两个吸收峰随时间推移不断增强[图2(f)].以上实验结果表明,MH NPs兼具类GPx、CAT和POD的多重催化活性,能够通过消耗细胞抗氧化剂GSH和生成强毒性•OH的“双重途径”破坏细胞的氧化还原平衡并引发严重的过氧化损伤,具有强力杀伤肿瘤细胞的潜在应用价值.
图2
图2
MH NPs的类酶催化性能. (a) MH NPs催化过程示意图;(b) GSH与不同浓度MH NPs(12.5、25、50、100和200 μg·mL-1)反应后DTNB溶液的紫外-可见吸收光谱和照片;(c) H2O2与不同浓度MH NPs(0、10 、20 、30 、40和50 μg·mL-1)反应O2随时间的生成情况;(d) H2O2分别与MH NPs(90 μL, 25 μg·mL-1)、Fe2+和H2O反应的电子顺磁共振谱图;(e)不同反应体系[TMB、TMB + H2O2和TMB + H2O2 + MH NPs(10 μL, 0.1 mg·mL-1)]的紫外-可见吸收光谱;(f)反应体系[TMB + H2O2 + MH NPs(10 μL, 0.1 mg·mL-1)]在不同时间的紫外-可见吸收光谱
Fig. 2
The enzyme-like catalytic performance of MH NPs. (a) Schematic illustration of catalytic process of MH NPs; (b) UV-vis absorption spectra and photographs of DTNB after reaction of GSH with MH NPs solution at different concentrations (12.5, 25, 50, 100, and 200 μg·mL-1) ; (c) The generation of O2 over time after the reaction of H2O2 with MH NPs of different concentrations (0, 10, 20, 30, 40 and 50 μg·mL-1) ; (d) ESR spectra of H2O2 reacting with MH NPs (90 μL, 25 μg·mL-1), Fe2+ and H2O, respectively; (e) UV-vis absorption spectra of different reaction systems [TMB, TMB + H2O2, TMB + H2O2 + MH NPs (10 μL, 0.1 mg·mL-1)]; (f) UV-vis absorption spectra of reaction system [TMB + H2O2 + MH NPs (10 μL, 0.1 mg·mL-1)] at different times
2.2.2 光热、光声性能
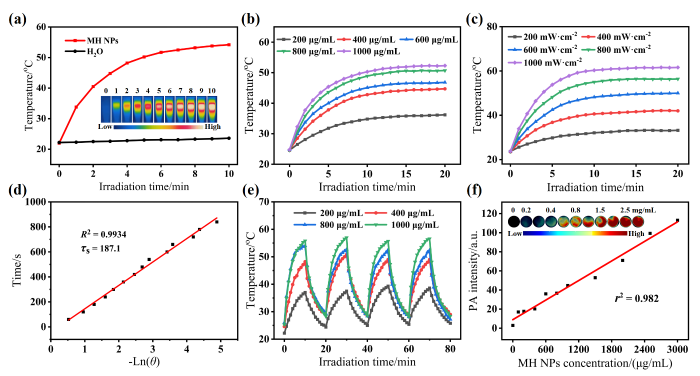
本研究还发现MH NPs具有良好的光热转换性能,当使用功率密度为500 mW/cm2的808 nm激光照射浓度为1 mg·mL-1的MH NPs溶液10 min后,溶液温度上升了25.1 ℃,而纯水的温度几乎保持不变[图3(a)]. 进一步研究发现,在808 nm激光照射下,MH NPs溶液的最高温度与溶液的浓度及激光功率密度有关:溶液浓度和激光功率密度越高,升温幅度与最高温度越高[图3(b)、(c)].基于后续测定可知,MH NPs的光热转换效率约为48.84 %[图3(d)].此外,在多次激光照射循环测试中,不同浓度的MH NPs溶液在温度变化上均保持良好的一致性[图3(e)],说明MH NPs具有较好的光热稳定性与可重复使用性.考虑到MH NPs良好的光热性能,我们进一步研究了其光声成像效果.通过采集不同浓度MH NPs溶液的光声图像发现,随着溶液浓度的增大,MH NPs的光声信号逐步增强,并与浓度呈现线性相关性[图3(f)],上述结果表明MH NPs具备应用于活体光声成像的潜力.
图3
图3
MH NPs的光热和光声性能. (a)近红外激光(808 nm激光,500 mW·cm-2)照射下MH NPs溶液(500 μg·mL-1)和H2O的升温曲线和红外热像图;(b) 808 nm激光(500 mW·cm-2)照射下不同浓度MH NPs溶液的温度变化曲线;(c)不同功率密度808 nm激光照射下MH NPs溶液(500 μg·mL-1)的温度变化曲线;(d) MH NPs光热转换效率的计算;(e) 808 nm激光(500 mW·cm-2)照射循环下MH NPs溶液的温度变化曲线;(f)光声(PA)信号强度与MH NPs溶液浓度的相关性以及不同浓度MH NPs溶液的光声图像
Fig. 3
The photothermal and photoacoustic performance of MH NPs. (a) The heating curve and infrared thermographs of MH NPs solution (500 μg·mL-1) and H2O under NIR laser irradiation (808 nm laser, 500 mW·cm-2); (b) Temperature change curves of MH NPs solutions with various concentrations irradiated by 808 nm laser (500 mW·cm-2); (c) Temperature change curves of MH NPs solution (500 μg·mL-1) irradiated by 808 nm laser of different power densities; (d) Calculation of the photothermal conversion efficiency of MH NPs; (e) Temperature change curves of MH NPs solutions under the cyclic irradiation of 808 nm laser (500 mW·cm-2); (f) Correlation of the photoacoustic signal intensity with the concentrations of MH NPs solutions and photoacoustic images of MH NPs solutions at different concentrations
2.2.3 磁共振成像能力
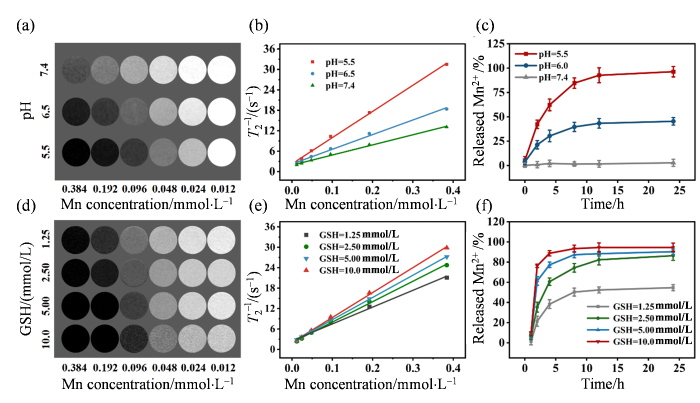
肿瘤细胞通常具有旺盛的能量需求和特殊的氧化应激状态,其独特的糖酵解代谢模式使得肿瘤微环境(Tumor microenvironment,TME)中H+和GSH含量较高[37,38].理想的MRI造影剂应对正常和肿瘤组织之间的微小差异高度敏感,并能表现出显著的反应差别[39].基于含锰或铁的纳米颗粒被用作MRI造影剂的研究成果[40
图4
图4
MH NPs的T2加权磁共振成像能力. (a)不同pH条件下MH NPs的T2 MRI图像;(b)不同pH条件下MH NPs的横向弛豫率与锰浓度之间的线性关系;(c)不同pH(pH = 7.4、6.5和5.5)条件下,MH NPs中Mn2+的体外释放曲线;(d) MH NPs与不同浓度GSH反应后的T2 MRI图像;(e) 不同GSH条件下,MH NPs的横向弛豫率与锰浓度之间的线性关系;(f)与不同浓度GSH反应后,MH NPs中Mn2+的体外释放曲线
Fig. 4
The T2-weighted MR imaging capability of MH NPs. (a) T2 MRI images of MH NPs at various pH conditions; (b) The linear relationship for the transverse relaxation rate of MH NPs as a function of Mn concentration under different pH conditions; (c) In vitro release curve of Mn2+ from MH NPs at various pH conditions (pH = 7.4, 6.5, and 5.5); (d) T2 MRI images of MH NPs after reaction with various concentrations of GSH; (e) The linear relationship for the transverse relaxation rate of MH NPs as a function of Mn concentration under different GSH conditions; (f) In vitro release curve of Mn2+ from MH NPs after reaction with various concentrations of GSH
2.3 MH NPs体外抗肿瘤研究
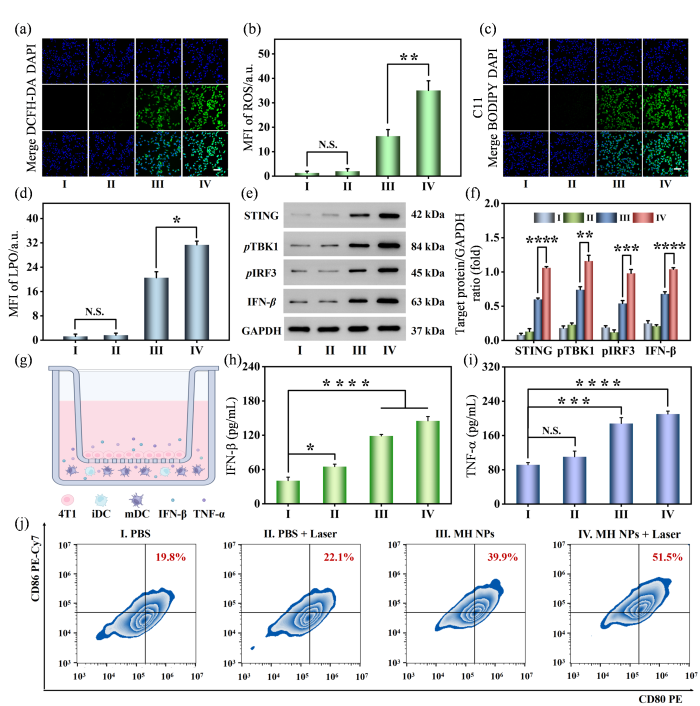
已有研究表明,光热效应可显著增强纳米材料的类酶活性[46,47].在光热作用的辅助下,MH NPs预计可通过消耗GSH和生成ROS两种途径引发更剧烈的过氧化损伤风暴以杀伤肿瘤细胞.为验证这一推测,我们首先通过CCK-8实验检测了MH NPs对BEAS-2B细胞和4T1细胞活性的影响.从实验结果来看,MH NPs处理和MH NPs + 808 nm激光处理几乎不会对BEAS-2B细胞的活性产生影响[附件材料图S3(a)],相比之下,MH NPs对4T1细胞产生了明显的毒性作用,当MH NPs中血红素的浓度为50 μg·mL-1时,4T1细胞的存活率下降至40.01%.施加808 nm激光(功率密度为1 000 mW•cm-2),4T1细胞的细胞活性进一步降低至28.36%[附件材料图S3(b)].另外,我们还发现孵育时间也会显著影响MH NPs对4T1细胞的毒性作用,当孵育时间超过12小时,4T1细胞的细胞活性降至50 %以下[附件材料图S3(c)].接下来,我们使用ROS荧光探针(DCFH-DA)对不同处理后的4T1细胞进行染色以验证MH NPs是否能诱导肿瘤细胞产生ROS.结果显示,对照组和单独接受808 nm激光照射组的4T1细胞几乎无明显绿色荧光,而MH NPs处理的细胞中显现出ROS的特征性绿色荧光,其荧光信号强度约为对照组的12.5倍.进一步施加808 nm激光照射后,4T1细胞中ROS探针的荧光强度相比于MH NPs单独处理组提高了约113%,增强至对照组的26.6倍[图5(a)、(b)].随后,我们还使用脂质过氧化(LPO)荧光探针(C11 BODIPY581/591)评估不同处理后4T1细胞的脂质过氧化损伤情况.如图5(c)、(d)所示,对照组和仅808 nm激光照射组的4T1细胞中几乎没有荧光,而MH NPs处理后的细胞则显现出LPO的特征性绿色荧光,其强度是对照组的16.2倍;在808 nm激光照射后,荧光信号进一步增强了约38%[图5(c)、(d)],这表明光热作用的确能增强MH NPs的类酶活性,引发更严重的LPO损伤.Western Blot结果也支持了上述推断:仅激光照射处理对GPX4的表达影响较小,而MH NPs处理组则观察到GPX4的表达明显下调.进一步施加808 nm激光照射,GPX4的表达进一步降低;但与铁死亡抑制剂(Fer-1)共同处理时,GPX4的表达又得到部分恢复(附件材料图S4).以上结果表明MH NPs具有出色的POD和GPx活性,在光热作用辅助下,MH NPs可在肿瘤细胞内形成剧烈的ROS风暴并诱发严重的铁死亡.
图5
图5
MH NPs介导光热作用增强的抗肿瘤催化治疗的体外评价. (a)不同处理后4T1细胞内ROS水平的共聚焦荧光图像(比例尺:100 μm);(b)不同处理后4T1细胞内ROS水平的半定量分析;(c)不同处理后4T1细胞内LPO水平的共聚焦荧光图像(比例尺:100 μm);(d)不同处理后4T1细胞内LPO水平的半定量分析;(e)不同处理后4T1细胞中STING通路相关蛋白表达的Western blot结果;(f)不同处理后4T1细胞中STING信号通路相关蛋白表达的半定量分析;(g)利用transwell系统探究不同处理对4T1细胞诱导的树突状细胞熟化情况示意图;(h)细胞上清液中IFN-β的表达水平;(i)细胞上清液中TNF-α的表达水平;(j)树突状细胞熟化水平的流式分析结果
Fig. 5
In vitro assessments of photothermal-enhanced anti-tumor catalytic therapy mediated by MH NPs. (a) Confocal fluorescence images of the cellular ROS level in 4T1 cells after different treatments (scale bar: 100 μm); (b) Semi-quantitative analysis of ROS level in 4T1 cells after different treatments; (c) Confocal fluorescence images of the cellular LPO level in 4T1 cells after different treatments (scale bar: 100 μm); (d) Semi-quantitative analysis of LPO level in 4T1 cells after different treatments; (e) Western blot assay of the STING signaling pathway-related protein expressed in 4T1 cells after different treatments; (f) Semi-quantitative analysis of the STING pathway-related protein expressed in 4T1 cells after different treatments; (g) The scheme of transwell system utilized to explore the maturation of dendritic cells induced by different treatments for 4T1 cells; (h) The expression level of IFN-β in the cell supernatant; (i) The expression level of TNF-α in the cell supernatant; (j) The level of mature dendritic cells measured by flow cytometry
此外,锰基纳米材料可通过释放Mn2+激活STING信号通路并促进树突状细胞成熟,从而增强肿瘤的免疫原性和机体的抗肿瘤免疫应答水平[48].基于此,我们首先通过Western blot实验检测了MH NPs处理后细胞中STING信号通路相关蛋白的表达情况.与PBS组相比,MH NPs处理组,特别是MH NPs + Laser处理组,STING信号通路相关蛋白STING、pTBK1、pIRF3和IFN-β的表达显著提高[图5(e)、(f)],这表明MH NPs可有效激活STING信号通路.与此同时,我们还在体外检测了细胞培养上清溶液中TNF-α和IFN-β的表达水平来评估MH NPs激活STING通路的能力[图5(g)].从分析结果来看,和PBS + Laser组相比,经MH NPs处理过的细胞上清溶液中IFN-β的表达水平更高,约为PBS组的2.94倍,辅以808 nm激光照射后,MH NPs处理的细胞上清溶液中细胞因子的表达水平进一步提高,IFN-β的表达水平提高至PBS组的3.59倍[图5(h)].与此同时,MH NPs相关处理组细胞的上清液中检测到了更高水平的TNF-α,MH NPs + Laser组TNF-α的表达水平约为PBS组的2.29倍[图5(i)],这些结果表明MH NPs可以通过激活肿瘤细胞的STING通路来激发抗肿瘤免疫反应.最后,我们通过流式细胞术评估了不同处理条件下树突状细胞的熟化情况.正如预期的那样,MH NPs组和MH NPs + Laser组树突状细胞的熟化水平显著高于前两组,MH NPs处理组树突状细胞的熟化水平约为对照组的2.95倍;在808 nm激光照射辅助下,其熟化水平进一步提高至对照组的4.87倍[图5(j)].以上所有实验结果表明,MH NPs + Laser诱导的ROS风暴不仅可以激活4T1细胞的STING信号通路,还能进一步促使树突状细胞成熟,增强机体的免疫应答,展现出良好的抗肿瘤免疫治疗潜力.
2.4 MH NPs活体成像
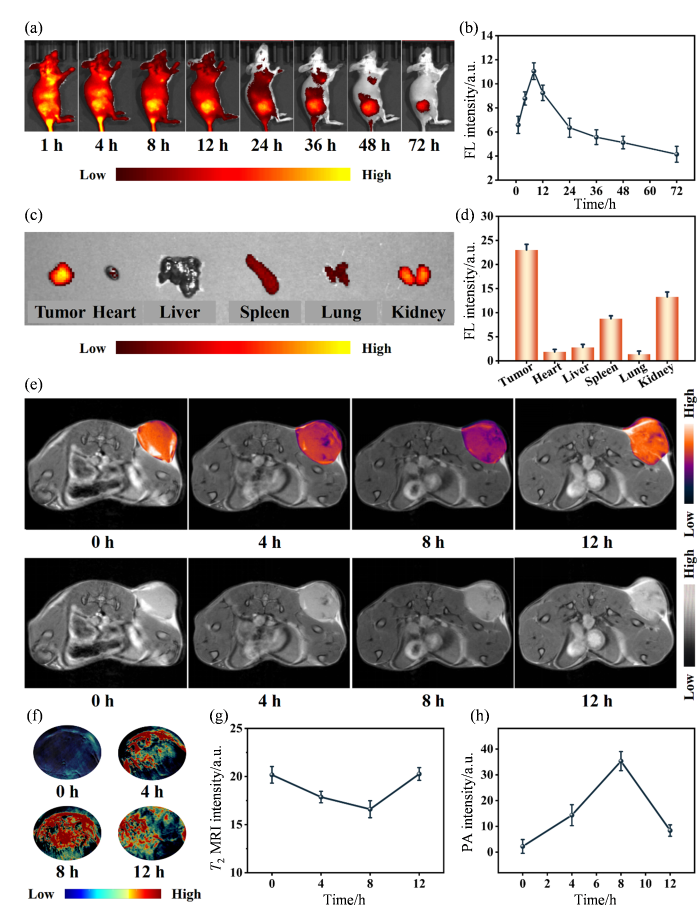
纳米颗粒在体内的代谢分布对于指导抗肿瘤治疗具有重要意义.首先,本研究利用活体荧光成像研究了MH NPs在4T1荷瘤小鼠体内的代谢分布情况.结果显示,MH@ICG NPs在静脉注射后逐渐向肿瘤部位富集,并在8 h达到峰值,这表明MH NPs可通过增强渗透与滞留(EPR)效应成功递送至肿瘤部位并且注射后8 h是在肿瘤部位施加808 nm激光(功率密度为800 mW•cm-2)照射的最佳时间点[图6(a)、(b)].此外,静脉注射72 h后,肿瘤部位仍然检测到较强荧光信号,且显著高于其他组织器官[图6(c)、(d)],这表明MH NPs可被有效地保留在肿瘤部位而不会在其他器官累积,避免了潜在的生物毒性风险.为进一步评估MH NPs对肿瘤的成像效果,静脉注射MH NPs后,我们采集了4T1荷瘤小鼠的T2 MRI图像和光声图像并进行了对比分析.MRI结果显示,注射MH NPs后,小鼠肿瘤部位的T2 MRI图像逐渐变暗且其信号随时间呈逐渐降低趋势,并在8 h降至最低值[图6(e)、(g)].光声成像结果亦与之相符:注射MH NPs后,小鼠肿瘤部位光声信号逐渐增强,并在8 h达到峰值[图6(f)、(h)].上述多模态成像结果表明,MH NPs能够高效富集于肿瘤部位并显著增强肿瘤组织与周围组织间的对比度,具备通过磁共振和光声成像对肿瘤进行可视化诊断的潜力.
图6
图6
MH NPs的活体生物分布和磁共振、光声成像. (a)静脉注射MH@ICG NPs不同时间点4T1荷瘤小鼠的活体荧光图像;(b)静脉注射MH@ICG NPs后不同时间点4T1荷瘤小鼠肿瘤部位的荧光变化曲线;(c)MH@ICG NPs静脉注射72 h后4T1荷瘤小鼠主要脏器的荧光图像;(d)静脉注射72 h后4T1荷瘤小鼠主要脏器中MH@ICG NPs的半定量分析;(e)静脉注射MH NPs后不同时间点4T1荷瘤小鼠的T2 MRI图像;(f)静脉注射MH NPs后不同时间点4T1荷瘤小鼠的光声图像;(g)静脉注射MH NPs后不同时间点4T1荷瘤小鼠肿瘤部位T2 MRI信号变化曲线;(h)静脉注射MH NPs后不同时间点4T1荷瘤小鼠肿瘤部位光声信号变化曲线
Fig. 6
In vivo biodistribution and MRI & PAI performance of MH NPs. (a) In vivo fluorescence images of 4T1 tumor-bearing mice at different time points after intravenous injection with MH@ICG NPs; (b) The fluorescence variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH@ICG NPs; (c) Fluorescence images of major organs of 4T1 tumor-bearing mice after intravenous injection with MH@ICG NPs for 72 h; (d) Semi-quantitive analysis of MH@ICG NPs in major organs of 4T1 tumor-bearing mice after intravenous injection with MH@ICG NPs for 72 h; (e) T2 MRI images of 4T1 tumor-bearing mice after intravenous injection with MH NPs at different time points; (f) Photoacoustic images of tumor sites in 4T1 tumor-bearing mice after intravenous injection with MH NPs at different time points; (g) The T2 MRI signal variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH NPs; (h) The photoacoustic signal variation curves of tumor sites in 4T1 tumor-bearing mice at different time points after intravenous injection with MH NPs
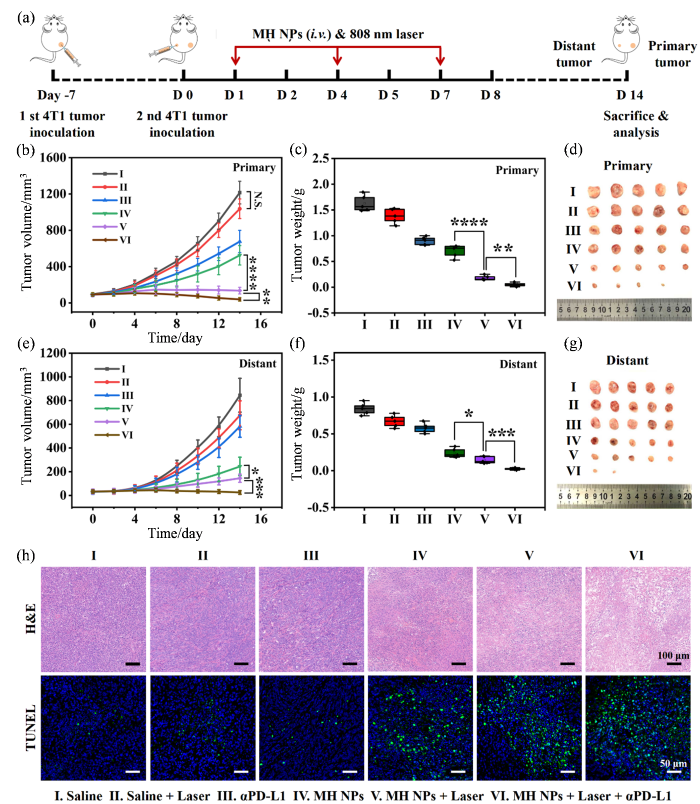
2.5 MH NPs活体抗肿瘤研究
基于MH NPs出色的多重酶样催化活性,我们进一步在双侧荷瘤小鼠模型上研究了光热促进MH NPs介导的催化型治疗协同αPD-L1的抗肿瘤治疗效果.治疗实验分为6个组别(I. Saline;II. Saline + Laser;III. αPD-L1;IV. MH NPs;V. MH NPs + Laser;VI. MH NPs + Laser + αPD-L1),并按照图7(a)所示流程图进行治疗.如图7(b-g)所示,单独808 nm激光(功率密度为800 mW•cm-2)照射对肿瘤生长的抑制作用有限;单独注射αPD-L1也仅能轻微抑制肿瘤生长.相比之下,基于MH NPs的治疗显著抑制了肿瘤的生长:在治疗结束时,MH NPs组的平均肿瘤体积为生理盐水组的43.1%,MH NPs + Laser组肿瘤的生长受到更强力的抑制,更值得注意的是,MH NPs + Laser + αPD-L1所介导的联合治疗使部分小鼠的肿瘤完全消退,最终的肿瘤平均体积仅为生理盐水组的3.2%.肿瘤组织切片的H&E染色和TUNEL染色结果进一步证实,上述联合治疗组表现出大量的坏死肿瘤细胞和最强的细胞凋亡水平[图7(h)].综合分析可知,MH NPs在光热作用辅助下可显著破坏肿瘤细胞的氧化还原稳态,诱发“毁灭性”的过氧化损伤;同时,MH NPs在TME中分解释放的Mn2+能激活肿瘤细胞的STING信号通路,促进树突状细胞成熟并提高机体的抗肿瘤免疫应答水平,协同增强αPD-L1的免疫治疗效果.
图7
图7
光热增强MH NPs介导的抗肿瘤催化免疫治疗联合αPD-L1对4T1移植瘤的治疗效果. (a)光热增强MH NPs介导的抗肿瘤催化免疫治疗联合αPD-L1的治疗示意图;(b, e)接受不同治疗的4T1荷瘤小鼠原发(Primary)和远端(Distant)肿瘤的生长曲线;(c, f)接受不同治疗的4T1荷瘤小鼠原发和远端肿瘤的重量;(d, g)接受不同治疗的4T1荷瘤小鼠原发和远端肿瘤的照片;(h)接受不同治疗的4T1荷瘤小鼠原发肿瘤的H&E和TUNEL组织染色分析
Fig. 7
Photothermal-enhanced MH NPs-mediated anti-tumor catalytic immunotherapy synergized with αPD-L1 against 4T1 tumor xenografts. (a) Schematic illustration of the photothermal-enhanced MH NPs-meidated anti-tumor catalytic immunotherapy synergized with αPD-L1; (b, e) Primary and distant tumor growth curves of the 4T1 tumor-bearing mice with different treatments; (c, f) The weight of primary and distant tumors in 4T1 tumor-bearing mice with different treatments; (d, g) Photographs of primary and distant tumors in 4T1 tumor-bearing mice with different treatments; (h) H&E and TUNEL staining histological analysis of primary tumor sections from 4T1 tumor-bearing mice with different treatments
值得注意的是,除了强大的抗肿瘤疗效,治疗策略的生理安全性亦至关重要.在治疗过程中,各组小鼠的体重无显著变化(附件材料图S5),主要器官的H&E染色结果未发现明显病理学异常(附件材料图S6),且血常规和血生化指标均处于正常范围(附件材料图S7).综上所述,基于MH NPs的抗肿瘤治疗方案在有效抑制肿瘤生长和转移的同时,具备良好的生物安全性,具有潜在的临床转化价值.
3 结论
本研究构建了基于Mn2+与内源性血红素的多功能纳米酶(MH NPs),以克服当前肿瘤免疫治疗中普遍存在的低免疫应答率及严重副作用等难题.一方面,该纳米酶的合成使用生物必需微量元素锰和内源性化合物血红素,具备良好的生物相容性和临床转化潜力.另一方面,MH NPs兼具有优异的GPx、POD和CAT等多重酶活性,可通过消耗GSH破坏肿瘤细胞的抗氧化防御体系,并生成过量ROS诱发过氧化损伤风暴,从而逆转ITME并激活机体的抗肿瘤免疫反应.在与免疫检查点抑制剂αPD-L1联合使用时,MH NPs能够强力抑制原发性与转移性肿瘤的生长.此外,MH NPs还能作为高效的MRI和PAI造影剂,实现对肿瘤的实时可视化成像.综上所述,基于天然化合物的多模式治疗策略为更安全、高效的肿瘤免疫疗法提供了新的思路,也预示了受生物系统启发的多功能纳米酶在未来肿瘤免疫治疗领域中具有广阔的应用前景.
利益冲突
无
附件材料附录
图S1 MH NPs的X射线光电子能谱
图S2 MH NPs在不同pH条件下的TEM图像
图S3 不同处理条件下BEAS-2B、4T1细胞的细胞存活率及不同处理条件下处理不同时间4T1细胞的细胞存活率
图S4 不同处理后4T1细胞中GPX4的表达水平
图S5 不同治疗方式下4T1荷瘤小鼠的体重变化曲线
图S6 各组小鼠主要脏器的H&E染色组织学分析
图S7 不同治疗方式下小鼠的血生化和血常规数据
参考文献
PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma
[J].
Improved survival with ipilimumab in patients with metastatic melanoma
[J].
Cancer immunotherapy comes of age
[J].
Combining immunotherapy and targeted therapies in cancer treatment
[J].
DOI:10.1038/nrc3237
PMID:22437869
[本文引用: 1]

During the past two decades, the paradigm for cancer treatment has evolved from relatively nonspecific cytotoxic agents to selective, mechanism-based therapeutics. Cancer chemotherapies were initially identified through screens for compounds that killed rapidly dividing cells. These drugs remain the backbone of current treatment, but they are limited by a narrow therapeutic index, significant toxicities and frequently acquired resistance. More recently, an improved understanding of cancer pathogenesis has given rise to new treatment options, including targeted agents and cancer immunotherapy. Targeted approaches aim to inhibit molecular pathways that are crucial for tumour growth and maintenance; whereas, immunotherapy endeavours to stimulate a host immune response that effectuates long-lived tumour destruction. Targeted therapies and cytotoxic agents also modulate immune responses, which raises the possibility that these treatment strategies might be effectively combined with immunotherapy to improve clinical outcomes.
The European medicines agency review of kymriah (tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma
[J].
Combining nanomedicine and immunotherapy
[J].
Nanoparticle-mediated remodeling of the tumor microenvironment to enhance immunotherapy
[J].
DOI:10.1021/acsnano.8b05893
PMID:30508378
[本文引用: 1]

Nanoscience has long been lauded as a method through which tumor-associated barriers could be overcome. As successful as cancer immunotherapy has been, limitations associated with the tumor microenvironment or side effects of systemic treatment have become more apparent. In this Review, we seek to lay out the therapeutic challenges associated with the tumor microenvironment and the ways in which nanoscience is being applied to remodel the tumor microenvironment and increase the susceptibility of many cancer types to immunotherapy. We detail the nanomedicines on the cutting edge of cancer immunotherapy and how their interactions with the tumor microenvironment make them more effective than systemically administered immunotherapies.
Combined nivolumab and ipilimumab or monotherapy in untreated melanoma
[J].
Nanotechnology for multimodal synergistic cancer therapy
[J].
DOI:10.1021/acs.chemrev.7b00258
PMID:29048884
[本文引用: 1]

The complexity, diversity, and heterogeneity of tumors seriously undermine the therapeutic potential of treatment. Therefore, the current trend in clinical research has gradually shifted from a focus on monotherapy to combination therapy for enhanced treatment efficacy. More importantly, the cooperative enhancement interactions between several types of monotherapy contribute to the naissance of multimodal synergistic therapy, which results in remarkable superadditive (namely "1 + 1 > 2") effects, stronger than any single therapy or their theoretical combination. In this review, state-of-the-art studies concerning recent advances in nanotechnology-mediated multimodal synergistic therapy will be systematically discussed, with an emphasis on the construction of multifunctional nanomaterials for realizing bimodal and trimodal synergistic therapy as well as the intensive exploration of the underlying synergistic mechanisms for explaining the significant improvements in synergistic therapeutic outcome. Furthermore, the featured applications of multimodal synergistic therapy in overcoming tumor multidrug resistance, hypoxia, and metastasis will also be discussed in detail, which may provide new ways for the efficient regression and even elimination of drug resistant, hypoxic solid, or distant metastatic tumors. Finally, some design tips for multifunctional nanomaterials and an outlook on the future development of multimodal synergistic therapy will be provided, highlighting key scientific issues and technical challenges and requiring remediation to accelerate clinical translation.
Renal-clearable PEGylated porphyrin nanoparticles for image-guided photodynamic cancer therapy
[J].
Tumor inhibition achieved by targeting and regulating multiple key elements in EGFR signaling pathway using a self-assembled nanoprodrug
[J].
Nanoscale melittin@zeolitic imidazolate frameworks for enhanced anticancer activity and mechanism analysis
[J].
Exudate absorbing and antimicrobial hydrogel integrated with multifunctional curcumin-loaded magnesium polyphenol network for facilitating burn wound healing
[J].
DOI:10.1021/acsnano.3c04556
PMID:37930078
[本文引用: 1]

Burns are among the most common causes of trauma worldwide. Reducing the healing time of deep burn wounds has always been a major challenge. Traditional dressings not only require a lengthy medical procedure but also cause unbearable pain and secondary damage to patients. In this study, we developed an exudate-absorbing and antimicrobial hydrogel with a curcumin-loaded magnesium polyphenol network (Cur-Mg@PP) to promote burn wound healing. That hydrogel was composed of an ε-poly-l-lysine (ε-PLL)/polymer poly(γ-glutamic acid) (γ-PGA) hydrogel (PP) and curcumin-loaded magnesium polyphenol network (Cur-Mg). Because of the strong water absorption property of ε-PLL and γ-PGA, Cur-Mg@PP powder can quickly absorb the wound exudate and transform into a moist and viscous hydrogel, thus releasing payloads such as magnesium ion (Mg) and curcumin (Cur). The released Mg and Cur demonstrated good therapeutic efficacy on analgesic, antioxidant, anti-inflammation, angiogenesis, and tissue regeneration. Our findings provide a strategy for accelerating burn wound healing.
A metal-polyphenol-coordinated nanomedicine for synergistic cascade cancer chemotherapy and chemodynamic therapy
[J].
Structural and molecular fusion MRI nanoprobe for differential diagnosis of malignant tumors and follow-up chemodynamic therapy
[J].
DOI:10.1021/acsnano.2c12874
PMID:36757738

Enhanced imaging techniques using contrast agents enable high-resolution structural imaging to reveal space-occupying lesions but rarely provide detailed molecular information. To this end, we report a structural and molecular fusion magnetic resonance imaging (MRI) nanoprobe for differential diagnosis between benign and malignant tumors. This fusion nanoprobe, termed FFT NPs, follows a working mechanism involving a -/-weighted magnetic resonance tuning effect (MRET) between a magnetic FeO core and a paramagnetic Fe-tannic acid (Fe-TA) shell. The FFT NPs with an "always-on" inert signal provide structural MRI (sMRI) contrast of tumors while affording an activated signal in the presence of ATP, which is overproduced during the rapid growth of malignant tumors to enable molecular MRI (mMRI) of tumor lesions. We propose the use of the ratiometric mMRI:sMRI intensity to assist in the differential diagnosis of malignant 4T1 tumors from benign L929 fibroblast tumors. Furthermore, the dissociated FFT NPs were found to be able to catalyze HO conversion in 4T1 tumors to generate excess reactive oxygen species (ROS) for chemodynamic therapy. The described fusion nanoprobe strategy enables the differential diagnosis of tumors from a combined spatial and molecular perspective with one-stop MRI imaging with potential applications in precision intervention.
Metal-phenolic network-enabled lactic acid consumption reverses immunosuppressive tumor microenvironment for sonodynamic therapy
[J].
DOI:10.1021/acsnano.1c08026
PMID:34661387
[本文引用: 1]

Nanomedicine has revolutionized cancer therapeutic strategies but has not completely changed the outcomes of tricky tumors that evolve a sophisticated immunosuppressive tumor microenvironment (TME) such as acidification. Here, a metal-phenolic network-based nanocomplex embedded with lactate oxidase (LOX) and a mitochondrial respiration inhibitor atovaquone (ATO) was constructed for immunosuppressive TME remodeling and sonodynamic therapy (SDT). In this nanocomplex, the sonosensitizer chlorin e6-conjugated polyphenol derivative can induce the generation of tumor lethal reactive oxygen species upon ultrasound irradiation. LOX served as a catalyst for intracellular lactic acid exhaustion, and ATO led to mitochondrial dysfunction to decrease oxygen consumption. This nanocomplex reversed the tumor immunosuppressive status by alleviating tumor hypoxia and acidic TME, achieving the characteristic enhancement of SDT and the inhibition of tumor proliferation and metastasis.
Mild photothermal therapy boosts nanomedicine antitumor efficacy by disrupting DNA mechanics damage repair pathways and modulating tumor
[J].
Tumor-targeting gene-photothermal synergistic therapies based on multifunctional polydopamine nanoparticles
[J].
Nanovesicles loaded with a TGF-β receptor 1 inhibitor overcome immune resistance to potentiate cancer immunotherapy
[J].
DOI:10.1038/s41467-023-39035-x
PMID:37328484
[本文引用: 1]

The immune-excluded tumors (IETs) show limited response to current immunotherapy due to intrinsic and adaptive immune resistance. In this study, it is identified that inhibition of transforming growth factor-β (TGF-β) receptor 1 can relieve tumor fibrosis, thus facilitating the recruitment of tumor-infiltrating T lymphocytes. Subsequently, a nanovesicle is constructed for tumor-specific co-delivery of a TGF-β inhibitor (LY2157299, LY) and the photosensitizer pyropheophorbide a (PPa). The LY-loaded nanovesicles suppress tumor fibrosis to promote intratumoral infiltration of T lymphocytes. Furthermore, PPa chelated with gadolinium ion is capable of fluorescence, photoacoustic and magnetic resonance triple-modal imaging-guided photodynamic therapy, to induce immunogenic death of tumor cells and elicit antitumor immunity in preclinical cancer models in female mice. These nanovesicles are further armored with a lipophilic prodrug of the bromodomain-containing protein 4 inhibitor (i.e., JQ1) to abolish programmed death ligand 1 expression of tumor cells and overcome adaptive immune resistance. This study may pave the way for nanomedicine-based immunotherapy of the IETs.© 2023. The Author(s).
Circulating immunotherapy strategy based on pyroptosis and STING pathway: Mn-loaded paclitaxel prodrug nanoplatform against tumor progression and metastasis
[J].
Biomimetic Fe3+ metal-phenolic networks enable DNAzyme and Cas9 RNP delivery for synergistic tumor ferroptosis-immunotherapy
[J].
Ferric-tannic nanoparticles inhibit early-stage hepatocarcinogenesis by activating tumor immune responses in rats
[J].
Noninvasively immunogenic sonodynamic therapy with manganese protoporphyrin liposomes against triple-negative breast cancer
[J].
Engineering dual catalytic nanomedicine for autophagy-augmented and ferroptosis-involved cancer nanotherapy
[J].
Curcumin doped zeolitic imidazolate framework nanoplatforms as multifunctional nanocarriers for tumor chemo/immunotherapy
[J].
Research progress on application of immune checkpoint inhibitors combined with curcumin in tumor treatment
[J].
免疫检查点抑制剂联合姜黄素在肿瘤治疗中的应用研究进展
[J].
Anti-tumor effect of curcumin on human colorectal cancer SW-620 cells
[J].
姜黄素对人结直肠癌SW-620细胞抗肿瘤效果的研究
[J].
Study on the effect of dihydroartemisinin on ferroptosis in ovarian cancer cells by regulating SLC7A11/GPX4 signaling pathway
[J].
双氢青蒿素通过调节SLC7A11/GPX4信号通路诱导卵巢癌细胞铁死亡
[J].
Research of dihydroartemisinin on anti-colorectal cancer by regulating MAPK/PI3K/Akt signaling pathway
[J].
双氢青蒿素通过调控MAPK/PI3K/Akt信号通路抗结直肠癌作用研究
[J].
Study on the nano-drug delivery system and anti-tumor mechanism of artemisinin and its derivatives
[J].
青蒿素及其衍生物纳米药物递送系统和抗肿瘤机制研究
[J].
Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism
[J].
DOI:10.1021/nn1029586
PMID:21218851
[本文引用: 1]

This paper demonstrated for the first time a simple wet-chemical strategy for synthesizing hemin-graphene hybrid nanosheets (H-GNs) through the π-π interactions. Significantly, this new material possesses the advantages of both hemin and graphene and exhibits three interesting properties. First, H-GNs have intrinsic peroxidase-like activity, which can catalyze the reaction of peroxidase substrate, due to the existence of hemin on the graphene surface. Second, their dispersion follow the 2D Schulze-Hardy rule, that is to say, the coagulation of H-GNs in electrolyte solution results from the interplay between van der Waals attraction and electric double-layer repulsion. Third, H-GNs exhibit the ability to differentiate ss- and ds-DNA in optimum electrolyte concentration, owing to the different affinities of ss- and ds-DNA to the H-GNs. On the basis of these unique properties of the as-prepared H-GNs, we have developed a label-free colorimetric detection system for single-nucleotide polymorphisms (SNPs) in disease-associated DNA. To our knowledge, this is the first report concerning on SNPs detection using functionalized graphene nanosheets. Owing to its easy operation and high specificity, it was expected that the proposed procedure might hold great promise in the pathogenic diagnosis and genetic diseases.
A hemin/G-quadruplex acts as an NADH oxidase and NADH peroxidase mimicking DNAzyme
[J].
G-quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy
[J].
DOI:10.1021/acs.nanolett.8b02732
PMID:30303384
[本文引用: 1]

Photodynamic therapy (PDT) is a light-triggered therapy used to kill cancer cells by producing reactive oxygen species (ROS). Herein, a new kind of DNA nanostructure based on the coordination between calcium ions (Ca) and AS1411 DNA G quadruplexes to form nanoscale coordination polymers (NCPs) is developed via a simple method. Both chlorine e6 (Ce6), a photosensitizer, and hemin, an iron-containing porphyrin, can be inserted into the G-quadruplex structure in the obtained NCPs. With further polyethylene glycol (PEG) modification, we obtain Ca-AS1411/Ce6/hemin@pHis-PEG (CACH-PEG) NCP nanostructure that enables the intranuclear transport of photosensitizer Ce6 to generate ROS inside cell nuclei that are the most vulnerable to ROS. Meanwhile, the inhibition of antiapoptotic protein B-cell lymphoma 2 (Bcl-2) expression by AS1411 allows for greatly improved PDT-induced cell apoptosis. Furthermore, the catalase-mimicking DNAzyme function of G-quadruplexes and hemin in those NCPs could decompose tumor endogenous HO to in situ generate oxygen so as to further enhance PDT by overcoming the hypoxia-associated resistance. This work develops a simple yet general method with which to fabricate DNA-based NCPs and presents an interesting concept of a nanoscale drug-delivery system that could achieve the intranuclear delivery of photosensitizers, the down-regulation of anti-apoptotic proteins, and the modulation of the unfavorable tumor microenvironment simultaneously for improved cancer therapy.
Functionalized tumor-targeting nanosheets exhibiting Fe(II) overloading and GSH consumption for ferroptosis activation in liver tumor
[J].
Nanozyme as tumor energy homeostasis disruptor to augment cascade catalytic therapy
[J].
DOI:10.1021/acsnano.4c09982
PMID:39661982
[本文引用: 1]

Breaking the balance of the tumor microenvironment and reshaping it sustainably remain major challenges in lung cancer treatment. Here, a "tumor energy homeostasis disruptor", the CuO@Au nanozyme was developed, which exhibits excellent glucose oxidase-like activity, enabling it to be used for starvation therapy and as a mimic peroxidase for chemodynamic therapy (CDT), producing OH. CuO@Au nanozymes consume glucose at the tumor site to block the tumor's energy supply, produce HO continuously, and lower the pH to enhance the efficiency of CDT, initiating a cascade reaction that leads to a storm of reactive oxygen species (ROS). Furthermore, CuO@Au nanozyme consumes glutathione and reduces the expression of the SLC7A11 (CT) protein to decrease cancer cell uptake of cysteine, further enhancing the burst of ROS, resulting in lipid peroxidation in tumor cells and ultimately leading to ferroptosis. The excellent photothermal performance of CuO@Au can further enhance CDT. Additionally, CuO@Au nanozyme also has computed tomography (CT) and photothermal imaging capabilities. In conclusion, CuO@Au nanozymes, acting as tumor energy homeostasis disruptor, can effectively inhibit tumor growth and successfully achieve the synergistic effects of starvation therapy/CDT/photothermal therapy (PTT). This multifunctional nanozyme holds promise for providing valuable insights and therapeutic strategies for cancer treatment.
Sprayable hydrogel for pH-responsive nanozyme-derived bacteria-infected wound healing
[J].
Metabolites and the tumour microenvironment: from cellular mechanisms to systemic metabolism
[J].
DOI:10.1038/s42255-020-00317-z
PMID:33398194
[本文引用: 1]

Metabolic transformation is a hallmark of cancer and a critical target for cancer therapy. Cancer metabolism and behaviour are regulated by cell-intrinsic factors as well as metabolite availability in the tumour microenvironment (TME). This metabolic niche within the TME is shaped by four tiers of regulation: (1) intrinsic tumour cell metabolism, (2) interactions between cancer cells and non-cancerous cells, (3) tumour location and heterogeneity and (4) whole-body metabolic homeostasis. Here, we define these modes of metabolic regulation and review how distinct cell types contribute to the metabolite composition of the TME. Finally, we connect these insights to understand how each of these tiers offers unique therapeutic potential to modulate the metabolic profile and function of all cells inhabiting the TME.
The updated landscape of tumor microenvironment and drug repurposing
[J].
Nanoparticle-based activatable MRI probes for disease imaging and monitoring
[J].
Peroxidase-like active nanomedicine with dual glutathione depletion property to restore oxaliplatin chemosensitivity and promote programmed cell death
[J].
DOI:10.1021/acsnano.1c06777
PMID:35266697
[本文引用: 1]

The nanocatalytic activity of nanozymes provides a vision for tumor treatment. However, the glutathione (GSH)-related antioxidant defense system (ADS) formed on the basis of excessive GSH in the tumor microenvironment limits its catalytic activity. Here, dendritic mesoporous silica nanoparticles (DMSNs) were employed as nanocarrier; ultrasmall FeO nanoparticles, Mn ions, and glutaminase inhibitor Telaglenastat (CB-839) were subsequently integrated into large mesopores of DMSNs, forming DMSN/FeO-Mn@CB-839 (DFMC) nanomedicine. This nanomedicine exhibits peroxidase mimicking activities under acidic conditions, which catalyzes the decomposition of hydrogen peroxide (HO) into hydroxyl radical (OH). This also promotes the formation of lipid peroxides, which is required for ferroptosis. Furthermore, this nanomedicine can effectively deplete the existing GSH, thereby enhancing reactive oxygen species (ROS)-mediated tumor catalytic therapy. Moreover, the introduced CB-839 blocks the endogenous synthesis of GSH, further enhancing GSH depletion performance, which reduces the excretion of oxaliplatin (GSH-related resistance) from tumor cells, thereby restoring the chemical sensitivity of oxaliplatin. The dual GSH depletion property significantly weakens the GSH-related ADS and restores the chemical sensitivity of oxaliplatin, leading to the high DFMC-induced apoptosis and ferroptosis of tumor cells. Our developed nanomedicine based on integrated nanotechnology and clinical drug may aid the development of tumor treatment.
Ultrasmall ternary FePtMn nanocrystals with acidity-triggered dual-Ions release and hypoxia relief for multimodal synergistic chemodynamic/photodynamic/photothermal cancer therapy
[J].
Tumor microenvironment-activable manganese-boosted catalytic immunotherapy combined with PD-1 checkpoint blockade
[J].
DOI:10.1021/acsnano.2c06646
PMID:36441901

Immune checkpoint blockade (ICB) therapy has attracted widespread attention in cancer treatment. Due to the low immunogenicity and immune suppression state in the tumor microenvironment (TME), the therapeutic effects are only moderate. Herein, a TME-activable manganese-boosted catalytic immunotherapy is designed for synergism with ICB therapy to kill tumors efficiently. The tumor cell membrane (CM)-wrapping multienzyme-mimic manganese oxide (MnO) nanozyme termed CM@Mn showed intrinsic peroxidase and oxidase-like activities in an acidic TME. These activities can generate toxic hydroxyl (OH) and superoxide radicals (O) for tumor cell killing and evoking immunogenic cell death (ICD). Furthermore, the TME-responsive release of Mn directly promotes dendritic cell maturation and macrophage M1 repolarization, resulting in the reversal of an immunosuppressive TME into an immune-activating environment. Additionally, tumor hypoxia relief caused by catalase-like activity also contributes to the process of TME reversal. Finally, a robust tumor-specific T cell-mediated antitumor response occurs with the support of the PD-1 checkpoint blockade. The proliferation of primary and metastatic tumors was inhibited, and a long-term immune memory effect was induced. The therapeutic strategy outlined here may serve as a promising candidate for tumor-integrated treatment.
Water-soluble Mn(III)-porphyrins with high relaxivity and photosensitization
[J].
Manganese-based contrast agents for MRI
[J].
锰对比剂在MRI中的应用
[J].
Research progress of manganese(II)-based contrast agents in magnetic resonance imaging
[J].
锰(II)基造影剂在磁共振成像中的研究进展
[J].
Nanohole-array induced metallic molybdenum selenide nanozyme for photoenhanced tumor-specific therapy
[J].
DOI:10.1021/acsnano.3c05000
PMID:37713431
[本文引用: 1]

Deficient catalytic sensitivity to the tumor microenvironment is a major obstacle to nanozyme-mediated tumor therapy. Electron transfer is the intrinsic essence for a nanozyme-catalyzed redox reaction. Here, we developed a nanohole-array-induced metallic molybdenum selenide (MoSe) that is enriched with Se vacancies and can serve as an electronic transfer station for cycling electrons between HO decomposition and glutathione (GSH) depletion. In a MoSe nanohole array, the metallic phase reaches up to 84.5%, which has been experimentally and theoretically demonstrated to exhibit ultrasensitive HO responses and enhanced peroxidase (POD)-like activities for HO thermodynamic heterolysis. More intriguingly, plenty of delocalized electrons appear due to phase- and vacancy-facilitated band structure reconstruction. Combined with the limited characteristic sizes of nanoholes, the surface plasmon resonance effect can be excited, leading to the broad absorption spectrum spanning of MoSe from the visible to near-infrared region (NIR) for photothermal conversion. Under NIR laser irradiation, metallic MoSe is able to induce out-of-balance redox and metabolism homeostasis in the tumor region, thus significantly improving therapeutic effects. This study that takes advantage of phase and defect engineering offers inspiring insights into the development of high-efficiency photothermal nanozymes.
Dual nanozyme-driven PtSn bimetallic nanoclusters for metal-enhanced tumor photothermal and catalytic therapy
[J].
Self-propelled in situ polymerized nanoparticles activating the STING pathway for enhanced bladder cancer immunotherapy
[J].